About Novodiax
Novodiax is dedicated to advancing tissue-based diagnostics and immunoassays through innovations that deliver meaningful test results in less time. Our signature technology platforms include our proprietary polymer-based (pHRP) detection system and our new rapid Q-STAIN® X Autostainer. Based on this pHRP technology, we have developed a series of highly sensitive polymer conjugates that are delivered to the market via our branded: ihcDirect®, pHRP™ labeled ihc (plus product name), and Q-STAIN X with its corresponding QSX™ line of reagents.
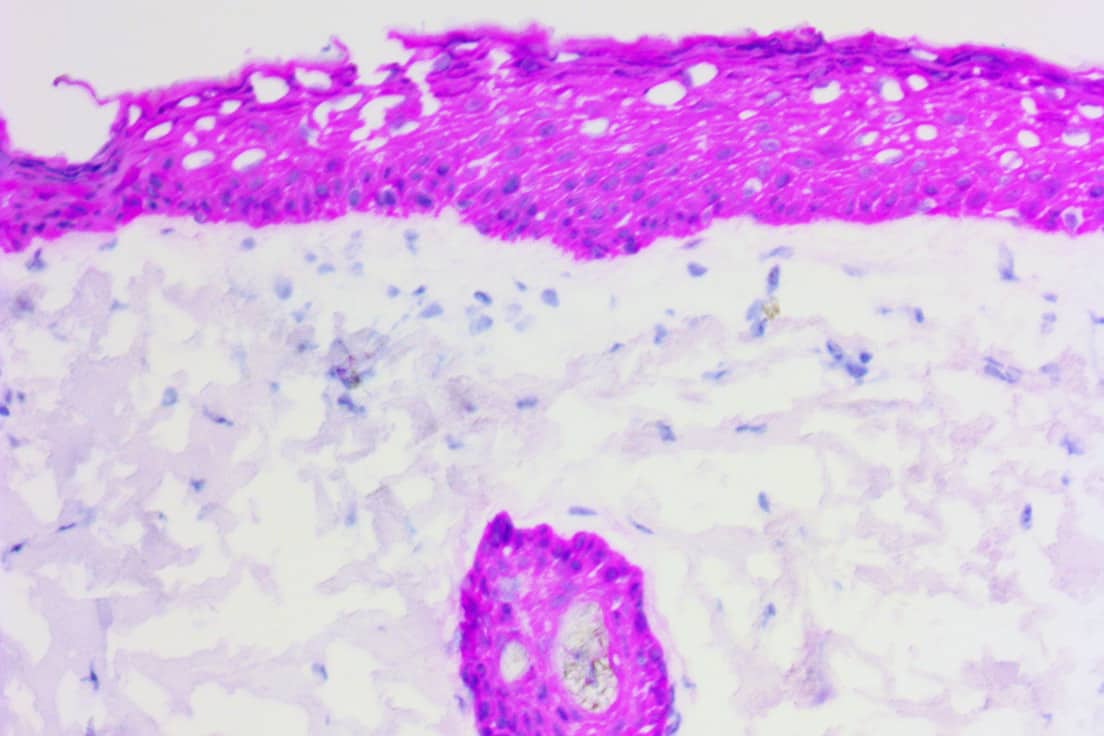
These novel histology product lines address unmet needs over a wide range of applications and markets such as rapid intraoperative tests for Mohs dermatological surgeries and surgical oncology as surgeons and pathologists interrogate and assess tissues and body fluids like those extracted through biopsies using fine needle aspirates (FNA) or frozen tissue sections from breast, lung or lymph nodes. These novel rapid tests may also facilitate a next generation of companion diagnostic tests that have distinct advantages over current companion diagnostic tests or that offer faster rule in/rule out of new drug candidates during drug discovery.
Contact us to learn moreihcDirect is a revolutionary IHC technology (patent pending) further advanced from our polymer detection systems. Some of the most commonly utilized diagnostic antibodies are directly conjugated to our polyHRP, resulting in a simpler and faster IHC procedure that minimizes intermediate steps. We have branded this new technology for IVD use as ihcDirect. These products have unique advantages over other IHC products in the market.
Intraoperative IHC diagnostics can only be practical if a procedure is less than 10 minutes. Our ihcDirect products have attained this level of speed, giving surgical oncologists the diagnostic information they need to improve patient care and reduce healthcare costs.
ihcPrime applies the same core technology to Research Use Only (RUO) products, giving clinical researchers the novel products and tools they need to advance clinical care. This novel platform also gives Novodiax and our collaborators the necessary tools to develop innovative companion diagnostic (CDx) products by directly labeling the therapeutic antibody, This results in reduced false positives from diagnostic tests, saving care givers and patients from unnecessary cost and human suffering.
Novodiax services for CDx antibody development fulfill at least three market needs: 1) determine the binding profile on cancer tissues in early development stage; 2) determine normal tissue cross-reactivity in late pre-clinical stage; and 3) companion diagnostics in clinical and market stages. By directly labeling therapeutic antibody, the issues of a) the unwanted non-specific bindings by endogenous biotin in two-step IHC; and b) the anti-human secondary antibody on human tissue in one-step IHC, are avoided. An IHC companion diagnostic tool can be readily developed from a supplied therapeutic antibody using our technology.
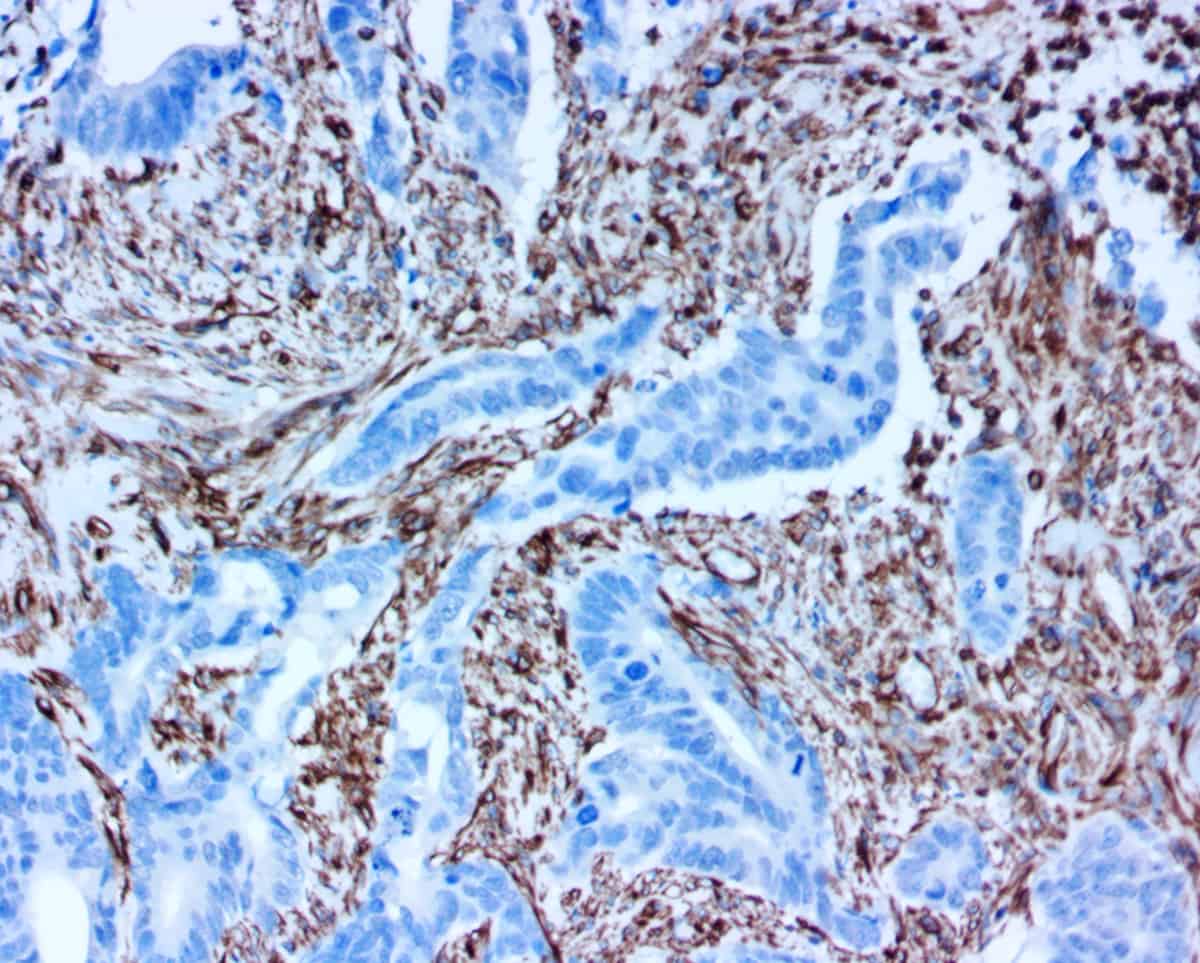
Q-STAIN® X products dramatically increase sensitivity and reduce time-to-results for IHC and ELISA immunoassays. These novel improvements reduce assay times in high throughput screening, and provide sensitivity improvements when performing tissue based immunohistochemistry in animal models and in clinical trials. Benefits of Q-STAIN® X include:
- High sensitivity: Massive amplifications by pHRP to boost signals in detecting low-abundant targets
- Low background: Amplifications without background from endogenous biotin
- Fast: Eliminates one incubation step
- Versatile: compatible with IHC, ELISA, and Western blotting
Our Commitment to Quality
The Novodiax Quality Policy is to develop and deliver safe, effective and reliable in-vitro diagnostic products through compliance with regulatory requirements, meeting customer needs, and maintaining the effectiveness of the quality management system.