Companion Diagnostics Development

Bench-to-bedside in less time
Would you like to streamline development of your IHC-based companion diagnostic (CDx) and speed up time-to-market? We can provide you with a rapid, sensitive IHC technology and custom support to help make that happen.
Leverage our ihcDirect® technology, accelerate CDx development
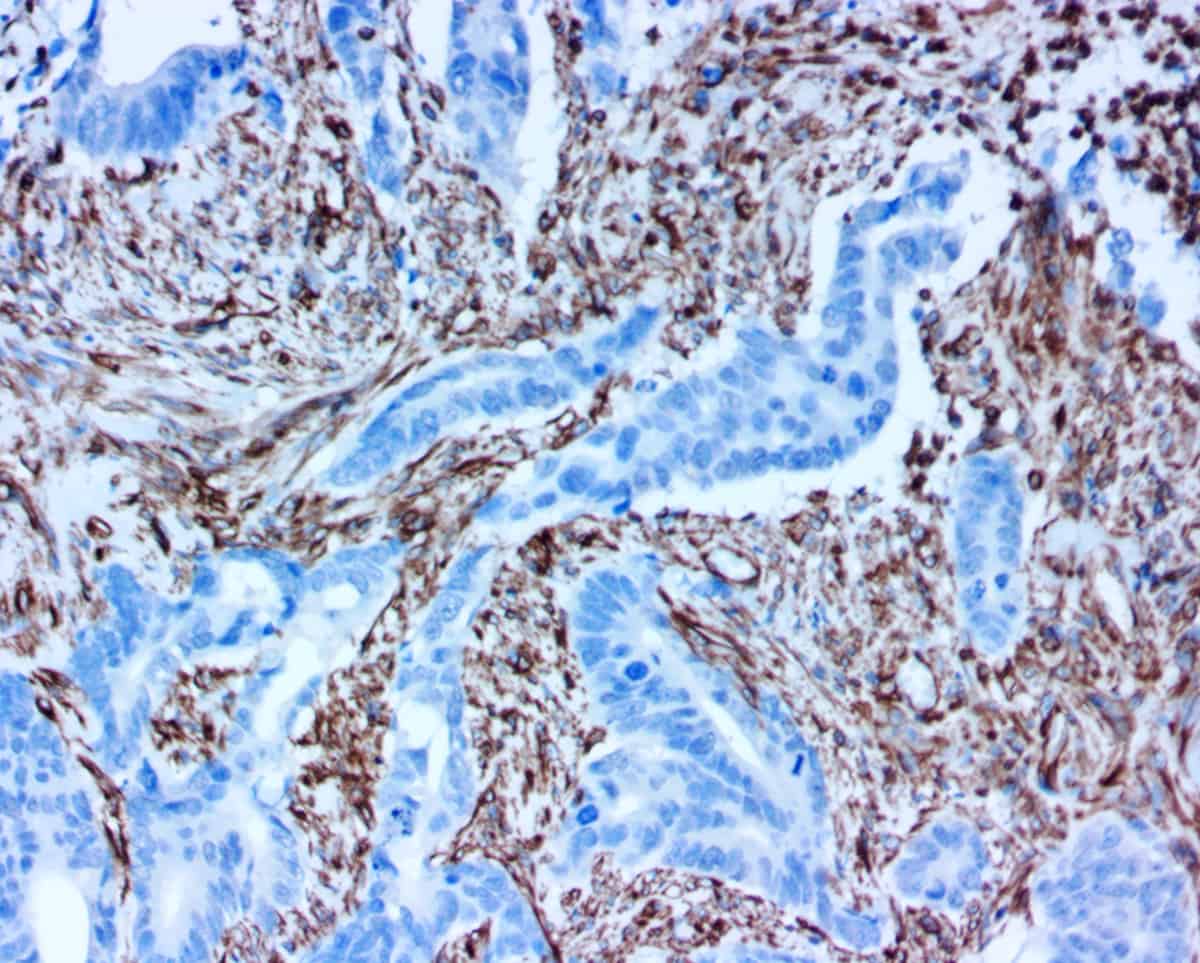
Use the Novodiax polymerized horseradish peroxidase (polyHRP) signal amplification technology (ihcDirect) and conjugate it directly to your humanized antibody-of-choice to enable rapid, sensitive IHC testing. Jumpstart your early humanized antibody-to-human tissue testing or ADME/toxicology studies to fail candidates early.
Current methods of CDx development force pharmaceutical antibody developers to modify the primary antibody in order to gain sensitivity and to fit into the current schemes of FFPE testing. Direct conjugation is better because your antibodies can be conjugated directly onto our pHRP system in an unmodified form. Thus, this next generation of immunoassay test saves you time, eliminates bias, and reduces the potential for cross-reactivity with secondary antibodies.
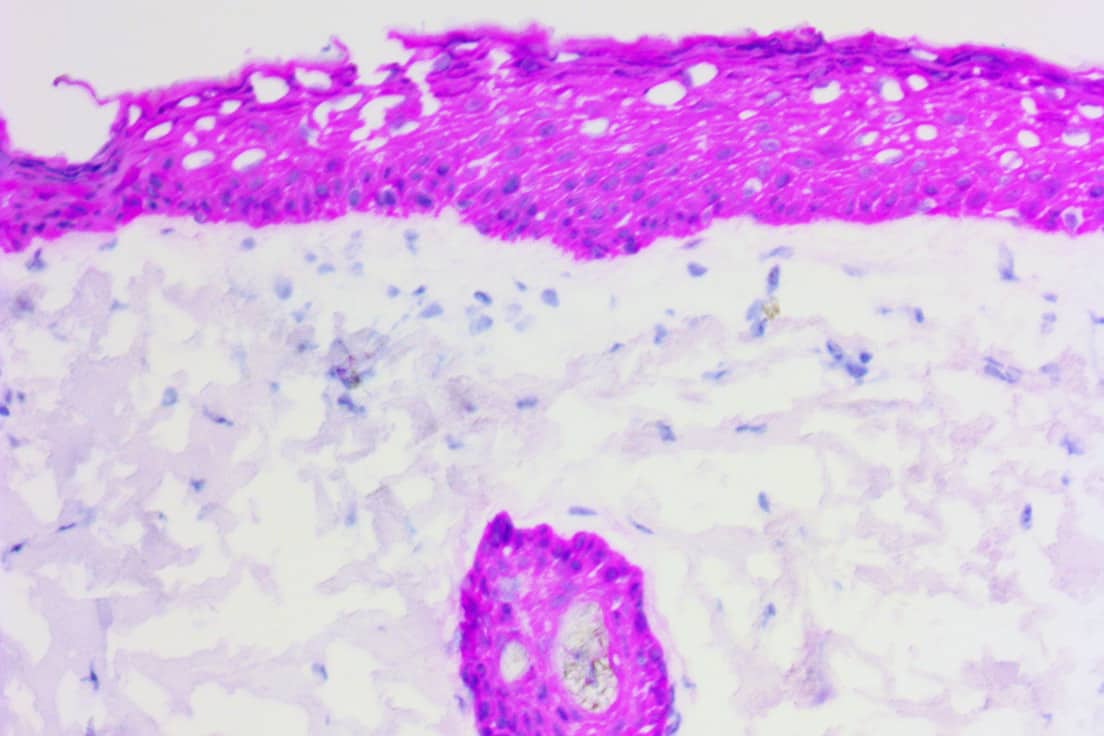
Our sensitive antigen detection system is perfect for rapid, high-precision IHC and ELISA testing and holds potential for future cytology test protocols. This approach can thus speed up development (biomarker identification, clone selection, validation and profiling, tissue cross reactivity testing, etc.) and provides high quality IHC results in as little as 10 minutes using frozen tissues!
We can provide custom support for your specific needs, or even partner with you through the entire development process.
Co-development of companion diagnostic and therapeutic may boost chances of success
Oncology drug development success rates are fairly low, with only an estimated 3.4% – 6.7% of candidates making it through clinical trials. But if you can more reliably predict how certain patients will respond to a therapy — by adopting a companion diagnostic earlier in your development process — your therapy may in turn benefit from a higher chance of successful outcomes and approval. Our ihcDirect technology and rapid IHC testing capabilities can help facilitate that synergy at every stage of development.
Applications
Our ihcDirect technology can be readily adapted to companion diagnostics for a wide variety of antibody therapies:
- Antibody Drug Conjugates (ADC)
- Antibody Dependent Cell-Mediated Cytotoxicity (ADCC)
- Signal Transduction blockage
- Immune check point blockage
- … and more
How we can help
- Conjugate your antibodies to our polyHRP
- Optimize in situ working conditions of the conjugated antibody using your positive and negative control tissues or cell lines
- Test binding profiles on human malignant tissues
- Test cross-reactivity on human normal tissues
- Deliver “ready to use” companion diagnostic for clinical trials, helping speed up your time-to-market
Let’s Discuss Your CDx Development Needs
If you are interested in collaborating with an experienced IVD IHC technology leader who can help you get to market with your companion diagnostic and therapeutic antibody faster, we would love to discuss such opportunities with you.