Posters and Presentations
Here, you will find a variety of posters that describe experiments, use-cases, and more for our in vitro diagnostic (IVD) and research products. Don’t see what you’re looking for? Contact Us
A Comparison between Manual and Automated Q-STAIN® X Immunostaining in Skin Cancer
Authors: Justin Jefferson, BS, MB(ASCP)CMHTLCM, Sarah Richardson, MS; Shuo Chen, PhD; David Hagebush, BS, BA; Song Zhao, MD, PhD, MPH
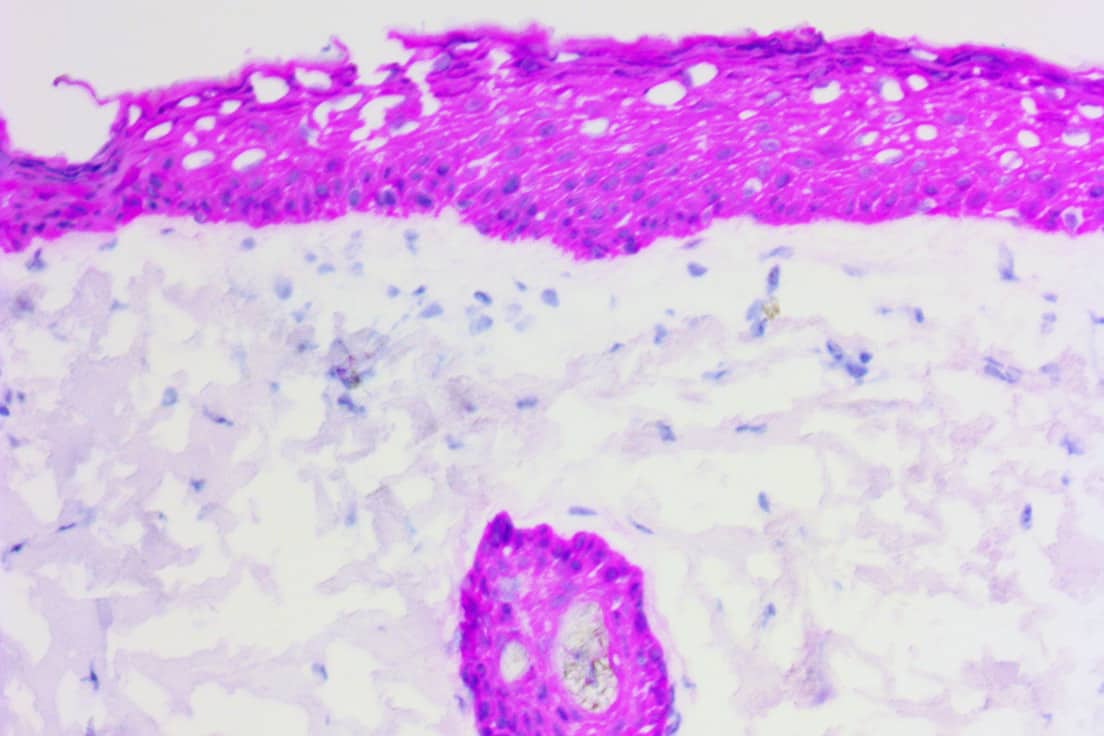
Purpose: Immunohistochemistry (IHC) demands for intraoperative surgery to support definitive surgical care have increased during a time of staff shortages in histology laboratories1,2. These added demands are driving the need to shift from manual IHC testing to automated IHC to increase the volume of slides tested, enhance the standardization of staining protocols while alleviating labor pressures and decreasing processing time2,3. Rapid automated IHC testing may help overcome insufficient human resources and help reduce human error while decreasing test turnaround times and improving overall laboratory efficiency. This combination of rapid automated IHC testing and quality results on frozen tissue sections may thereby benefit intraoperative surgeries such as Mohs micrographic surgery (MMS)4. Melanocytic markers such as Melanoma antigen recognized by T cells (MART-1) and SRY-related HMG-box 10 (SOX10) are often used to identify melanoma during extraction of malignant tissue during MMS5,6. MART-1 and SOX10 antibodies stain malignant melanocytes and normal background melanocytes that serve as internal controls. MART-1 is a cytoplasmic marker commonly used as an adjunct to Hematoxylin and Eosin (H&E) frozen sections to detect junctional and focal dermal melanocytic proliferation on frozen sections6. SOX10 is a nuclear marker and has historically been considered the standard for desmoplastic and spindle cell melanomas7,8. Cytokeratin 5 (CK5) is a high molecular weight keratin expressed in squamous cell epithelium, myoepithelial cells of the breast, mesothelium, and the basal cells of the prostate. CK5 is a rabbit monoclonal antibody (mAb) that binds specifically to basal and squamous cell carcinoma of skin, and it also binds to squamous cells of epidermis and hair out-root sheath cells, sebaceous glands, and basal cells of eccrine and apocrine ducts9. These properties make CK5 antibody a reliable marker for both basal cell (BCC) and squamous cell carcinoma (SCC) of skin9. In the present study, we aimed to demonstrate concordant staining results when comparing manual staining to Q-STAIN®X (QSX) autostainer results when using Novodiax (Hayward, CA) anti-MART-1, anti-SOX10, and anti-CK5 antibodies with polymerized Horseradish Peroxidase (poly-HRP) technology on frozen skin cancers (melanoma and non-melanoma). The staining results were reviewed and graded by an in-house pathologist to assess staining quality.
Summary: The rapid QSX autostainer turnaround time, consistent staining quality, and minimal need for operator interaction support its use for clinical intraoperative applications such as MMS. Results indicate the clinical utility of standardized and consistent automated IHC in intraoperative testing as seen with MART-1, SOX10, and CK5 to aid in rapid identification and removal of malignant tissues.
Demonstration of Rapid Immunocytochemistry: Resolve Molecular Targets in 10-20 Minutes
Authors: Shuo Chen, PhD; Susan Providenza; Ziman Cheng; Jianying (Kevin) Yang; Yan Zhang; Jianfu J. Wang, PhD; and Jin V. Wu, PhD
Background: The Novodiax ihcDirect® family of IVD products are capable of 10-minute immunohistochemistry (IHC) staining on frozen tissue sections1. The technology is derived from a proprietary platform of polymerized horseradish peroxidase (polyHRP) directly conjugated to a primary antibody. Such rapid IHC assays enable the timely intra-operative evaluation of resection margins (e.g. Mohs micrographic surgery2) as well as intra-operative differential diagnosis (e.g. breast cancer surgery3). Consequently, there is now a great deal of interest in fast and sensitive biomarker-based assays that could similarly enable the rapid on-site evaluation (ROSE) of cytology specimens to help determine sample adequacy and triage biopsy material appropriately without having to wait for timeconsuming cell block preparations. The objective of the present study is to begin to characterize novel applications of Novodiax’s ihcDirect polyHRPconjugated antibodies for rapid immunocytochemistry (ICC) staining on freshly prepared whole cell samples.

Use of Herceptin-Based IHC as a Potential CDx for Herceptin Treatment
Authors: Lijuan Wang, MD PhD, Yihong Wang, MD PhD, Song Zhao, MD PhD MPH, Yonghua Zhang PhD, Angelina Motiee, Zhiqing Zhang, PhD
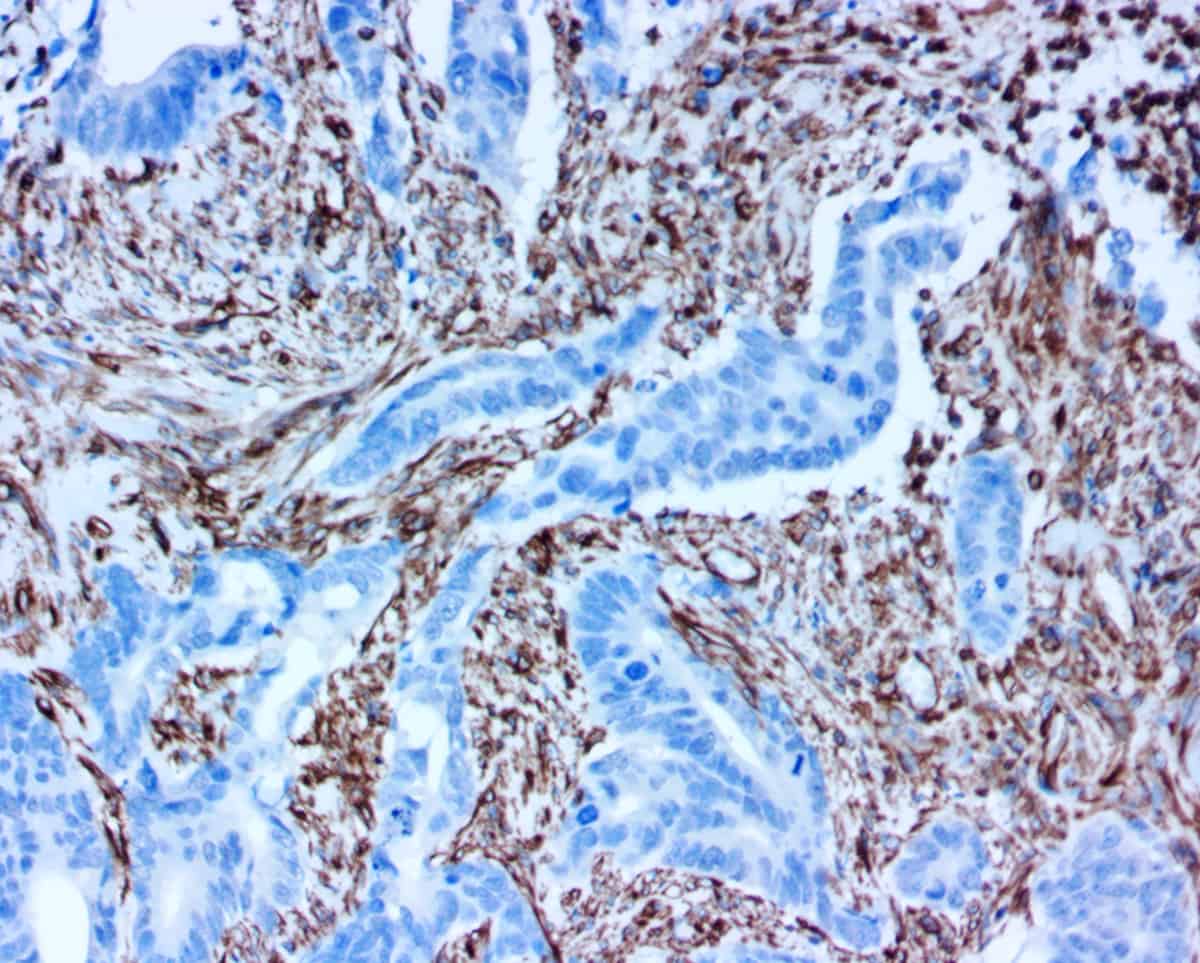
Background: Current ASCO and CAP guidelines require using FDA approved Her2 IHC or Her2 ISH tests to select Her2 positive patients for Herceptin therapy and that all Her2 IHC 2+ results must be confirmed by a Her2 ISH test. Combination of the two tests has reduced the false positive rate dramatically from about 20% by using Her2 IHC alone to about 6% which still represents a significant number of patients who are potentially treated with the expensive drug without therapeutic effect. With more Herceptin based therapies (e.g., ADC, CAR-T, immunotherapy) are available or will be available soon, a more reliable Her2 test is becoming critically important in selection of eligible Her2 positive patients for these therapies. An ideal companion test for Herceptin therapy should be a test that can directly detect Herceptin binding activity to patient’s cancer tissues. Novodiax has developed a simple Her2 IHC by directly labeling Herceptin with a unique polymerized HRP, namely ihcDirect-Herceptin, which can directly detect Herceptin binding activity on patient’s cancer tissues. This Her2 IHC does not require any secondary antibody or other detection steps that is needed in a “traditional IHC”. We report here a superior result by ihcDirect-Herceptin than two conventional Her2 IHC tests based on a large scale evaluation of 119 archived breast cancer tissues.

Rapid and sensitive multiplexing approach with Novodiax ihcDirect Technology
Authors: Shuo Chen, PhD; Zhiqing Zhang, PhD; Jianfu Wang, PhD
Background: Multiplex testing can optimize the use of precious specimens, provide better insights into the local microenvironment, reveal a better understanding of co-expression and spatial organization of biomarkers and/or distinguish different cells in blood samples. The Novodiax ihcDirect technology, in comparison to conventional immunohistochemistry (IHC), provides a simpler and faster test procedure. Novodiax uses a novel polyHRP-antibody detection system to improve both intraoperative IHC on frozen tissues as well as immunocytochemistry (ICC) on fresh cells. Tests for both processes can be completed in just 10 minutes while demonstrating superior detection sensitivity and maintaining low background across a wide variety of antibodies. Thus, the multiplex approach with Novodiax ihcDirect technology can be a powerful tool to investigate for hidden clues in cell and tissue samples in a rapid fashion. For instance, a triplex IHC/ICC on fresh tissue or cells can be finished within an hour. Recently, this technology has been applied to the analysis of blood samples in liquid biopsy for detecting circulating tumor cells using both colorimetric and fluorescent substrates.

Optimization of Rapid Immunohistochemical Stains on Frozen Tissue Sections
Authors: Haiyan Liu, MD, Song Q Zhao, Yonghua Zhang, PhD, Zhiqing Zhang, PhD, Fan Lin, MD, PhD
Background: Intraoperative frozen section consultation plays an important role in patient management. However, accurate diagnosis can sometimes be challenging. The application of rapid immunohistochemistry (IHC) on frozen sections, recently introduced by Novodiax, Inc. (Hayward, CA), can be useful when working on a difficult case. The aim of this study is to test and optimize the staining conditions for this rapid IHC technique using horseradish peroxidase (HRP) polymer labeled mouse anti-human AE1/AE3 antibody.

Development of a practical 10-min intraoperative IHC product line
Authors: Yonghua Zhang, PhD; Zhiqing Zhang, PhD; Jianfu Wang, PhD; Song Zhao, MD, PhD, MPH; Fan Lin, MD, PhD
Introduction: Intraoperative frozen section (FS) diagnoses are expected to be completed within 20 minutes (min). The conventional immunohistochemistry (IHC) has a very limited application because it requires at least 2-3 hours finishing the staining process. Numerous attempts have been made to develop a fast IHC on FS. However, all previous attempts have failed because these procedures were too complicated, costly, or unable to be applied to all important diagnostic markers. Using a unique polymerized horseradish peroxidase (pHRP), we successfully developed a 10-min IHC assay for the most commonly used antibodies on FS at room temperature.

Authors: Robert Glinert, MD and Song Q. Zhao, MD, Ph.D., MPH
Summary: In this study, a novel one-step CK5 direct immunohistochemistry (IHC) was used to map basal cell and squamous cell carcinoma on frozen section skin tissues during Mohs micrographic surgery (MMS). Total 64 frozen section skin specimens from 21 patients who have either basal cell carcinoma or squamous carcinoma were used for this study. The results showed that this one-step CK5 direct IHC can detect both basal cell(BCC) and squamous carcinoma(SCC) on skin frozen section tissues with minimal background stain. This one-step CK5 direct IHC method is simple to use, and it is ultra-fast comparing with current IHC methods. The entire procedures of this one-step CK5 direct IHC can be finished within 10 min with no need of expensive machine and it can be performed either at room temperature or on a slide warmer at 30°C. This novel one-step CK5 direct IHC method is potentially a valuable tool for accurately mapping tumor cells on frozen section skin tissue during MMS and to improve the outcome of surgical treatment of skin cancer.
Still have questions?
If you couldn't find what you were looking for and still have questions, don't hesitate to reach out.
Contact Us