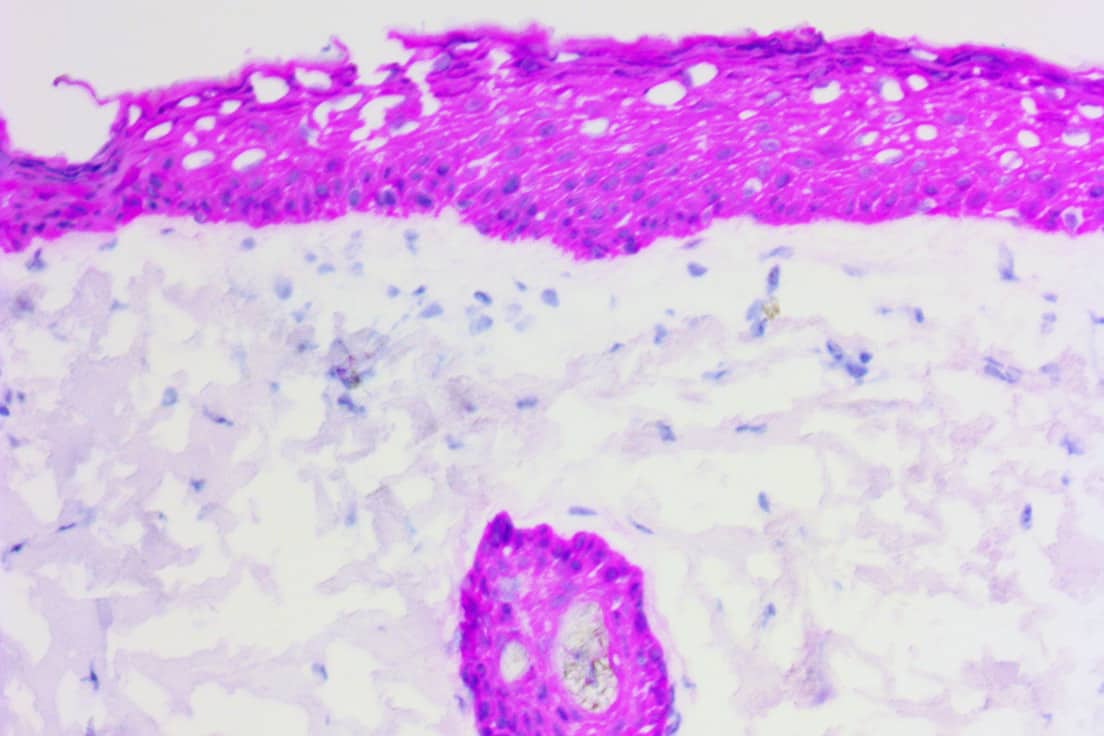
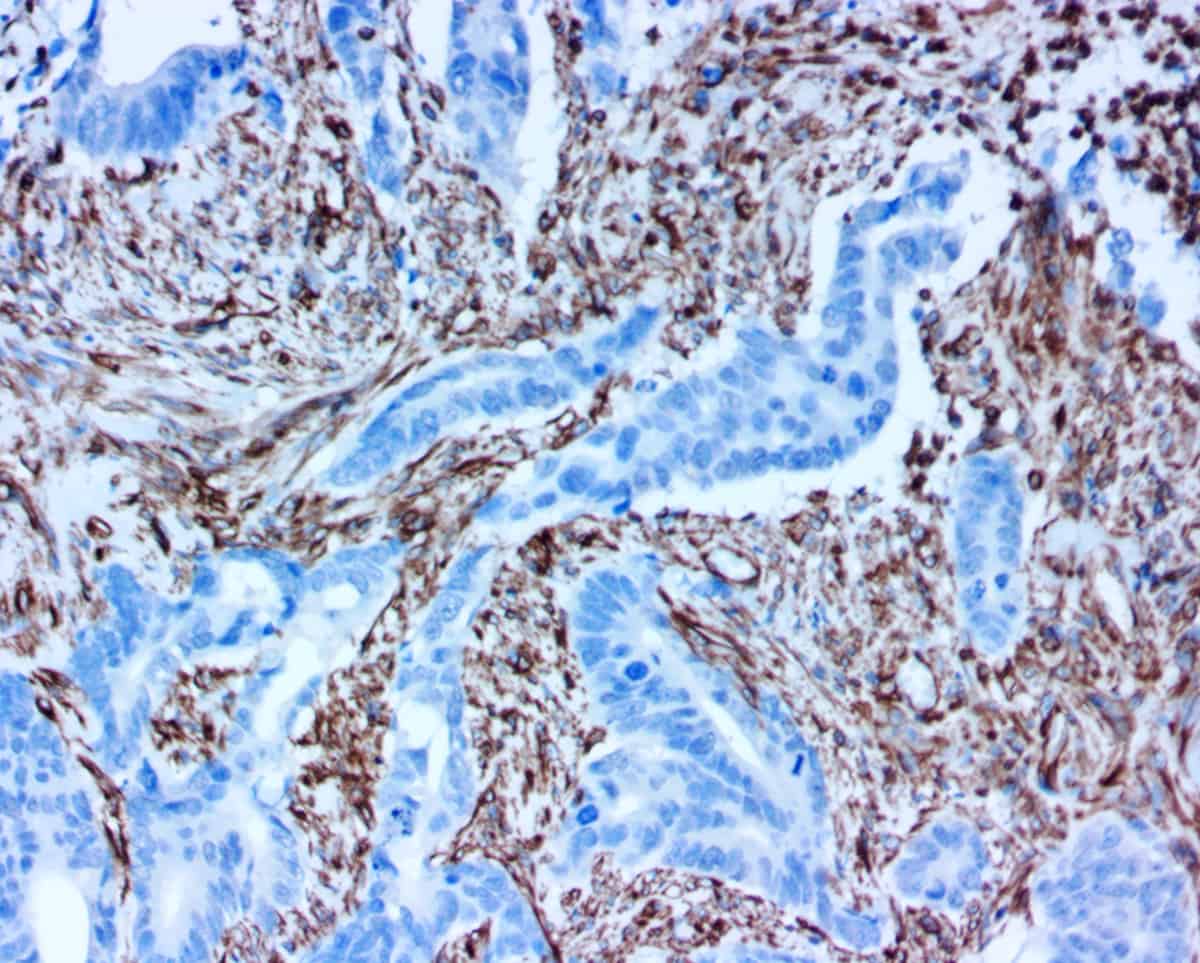
APPLICATIONS
ihcDirect® is the in vitro diagnostic brand name for products that utilize the exclusive Novodiax a polymerized HRP p(HRP) that is conjugated directly onto primary antibodies. This technology is further advanced from other polymer and non-polymer detection systems resulting in simpler and faster IHC test procedures with fewer intermediate steps and shorter time intervals within each step than standard IHC antibody test protocols.
Our novel polyHRP labeling method is a revolutionary technology (patent pending) that opens a spectrum of new applications.
Intraoperative IHC
Many publications have indicated that receiving lab results in “ideally less than 20 minutes” is highly desirable for a variety of intraoperative surgeries (eg sentinel lymph node assessments, breast, lung, brain, and thyroid cancers). These rapid immunoassay tests expand the information physicians have over hematoxylin and eosin (H&E) testing thereby helping the physician to make more informed decisions at the time of surgery. IHC is more sensitive than H&E testing alone and these results appear to be more binary, e.g. disease is present or absent from the tissues being tested and can thereby lead to improve medical outcomes. Intraoperative tests performed by IHC can be a highly valuable tool but can only be practical if the entire IHC procedure can be completed in a timely manner (~ 20 minutes).
Conventional IHC processes require hours to complete and are, therefore, impractical for intraoperative applications. H&E’s though a standard of practice today can also be inconclusive leading to delayed or compromised decision making during intraoperative surgery.
Patients are well served by a highly sensitive and rapid IHC method that uses fresh frozen tissues at the time of surgery.
With ihcDirect assays, information can be made available in minutes rather than days, so it may be possible to immediately impact the medical decisions being made, such as which mode of intervention or which therapy is chosen for the patient. Also, go-no-go decisions may be enabled such as do we need to take a couple of lymph nodes from this patient versus 10-12 nodes? Making quality decisions now vs. days later can greatly impact a hospital’s workflow of patients in the operating room and the number of repeat or follow-up surgeries required for a patient when a false-negative result occurs and is only discovered days after the original surgery.
Mohs Surgery
Success with Mohs Micrographic Surgery (MMS) depends on accurate mapping of the tumor, correct interpretation of the histopathological sections, and appreciation of aggressive tumor characteristics. Because H&E staining may present difficulties in interpretation of frozen sections, ihcDirect tests are being used to supplement these routine stains with greater sensitivity and specificity.
Adjunctive use of ihcDirect with H&E frozen sections enhances tissue interpretation and spares resection of additional tissue. The most common reason for recurrence of tumor after MMS is residual undetected tumor.
ihcDirect leads to facilitated surgical excision via MMS by reducing variable staining, high background or nonspecific staining, and turn-around time. Specifically, ihcDirect is useful in clearly delineating malignant cells present in dense inflammation, identifying perineural invasion and pagetoid spread in carcinomas.
ihcDirect has overcome technical obstacles that previously made traditional IHC tests for Mohs surgery unmanageable.
- First, IHC stains were initially developed for FFPE sections and not frozen sections. Consequently, there were problems with displacement of soluble antigens on frozen sections. ihcDirect kits are formulated and optimized specifically for 10 minute procedures when working with fresh frozen tissues.
- Second, the use of polyclonal antibodies led to decreased specificity because some antigens identified by polyclonal antibodies may also belong to normal tissue. All ihcDirect kits utilize monoclonal antibodies, maintaining antigen specificity and reducing backgrounds.
- And finally, incubation times required for traditional IHC stains are 60 minutes or more. ihcDirect procedures generate accurate high quality results in 10 minutes or less.
Additional Intraoperative Surgeries
There are many other current and potential opportunities for frozen tissue IHC tests. These include, but are not limited to:
- Sentinel Lymph Node (SLN) assessment
- Assessing surgical margins with melanoma
- Supporting decisions during organ transplantation
- Sclerosing tumors
- Simplifying tests in off hours (eg nights & weekends)
- Epithelial melanoma tests with transplantation procedures
- In support of a wide variety of biopsies and tissue extractions
Companion Diagnostics
Because of the direct conjugation of pHRP onto the therapeutic protein or antibody, Novodiax’s ihcDirect offers a completely new approach to companion diagnostic (CDx) testing. Direct labeling of THE therapeutic antibody carries huge benefits for the patient as well as the time requirements for developing the drug therapy. Using ihcDirect means that the best candidates can be chosen using human tissues and with little to no modification to the therapeutic candidate. The highly sensitive pHRP will also facilitate novel uses of the CDx for possible use in cytology, as well as traditional IHC testing.
Working towards a better Companion Diagnostic
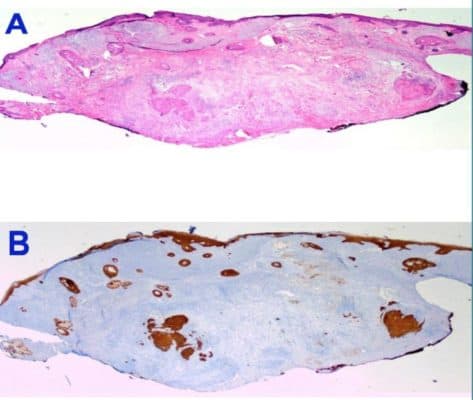
HER2 is overexpressed in about 20% of breast cancer and 30% of stomach cancer cases. Treating HER2 positive cancer patients with trastuzumab, a humanized therapeutic antibody, depends on the positive tests by either IHC or FISH. The two current tests have significant issues of discordance and inconsistencies between testing labs. The FDA has approved four mouse or rabbit antibody based IHC and multiple ISH products as companion diagnostic tools for HER2 therapies. After years of improvement, there are still about 6% false positive results that could lead to the ineffective adjuvant HER2-targeted therapy for 1 year and about 2% false negative that could lead to denial of trastuzumab treatment for a patient who could benefit from the therapy. An attractive strategy would be using the therapeutic antibody itself as a diagnostic tool to directly detect its binding target on cancer cells. However, applying humanized antibody on human human tissue by IHC method has always been challenging due to non-specific binding. Novodiax’s ihcDirect technology (patent pending) offer a new solution by labeling a therapeutic antibody directly with a unique and highly sensitive polyHRP.
In our pilot study, we have compared the performance of Novodiax’s polyHRP-trastuzumab on archived FFPE breast cancer tissues with the results of Dako’s HercepTest (IHC) and FISH that were generated in a CAP certified clinical and commercial labs. For more details, our USCAP poster can be found here: USCAP Herceptin POSTER – Final
ELISA
Conjugate your primary or secondary antibody with our polyHRP platform to:
- Optimize in-situ working conditions of the conjugated antibody with your positive and negative control tissues/cell lines
- Test the cross-reactivity of your antibodies on tissues/cell lines
- Increase sensitivity with ELISA based high throughput drug screening for increased throughput and improved pharmacology
- ELISA applications are for research use only