Press Release
Novodiax introduces ihcDirect® Pan-CK 4Abs, AE1/AE3, CEA, and CD45 reagents to growing list of 10-Minute IHC Assays for in-vitro diagnostic use.
Hayward, CA, August 16, 2018– Novodiax Inc., a leader in intraoperative immunohistochemistry (IHC) technology, announces the availability of four new ihcDirect staining kits for in vitro diagnostic (IVD) use: Pan-CK 4Abs, AE1/AE3, CEA and CD45 Assays. “These products demonstrate our continued drive to deliver meaningful 10-minute IHC test results on frozen tissues to improve patient care for hospitals and clinicians,” said Jianfu Wang, PhD, CEO of Novodiax. The Oncology assays can reduce surgeon wait times for histology results from hours to minutes, thus speeding the clinical interpretation of tissue staining patterns while often eliminating subsequent follow up visits and surgeries.
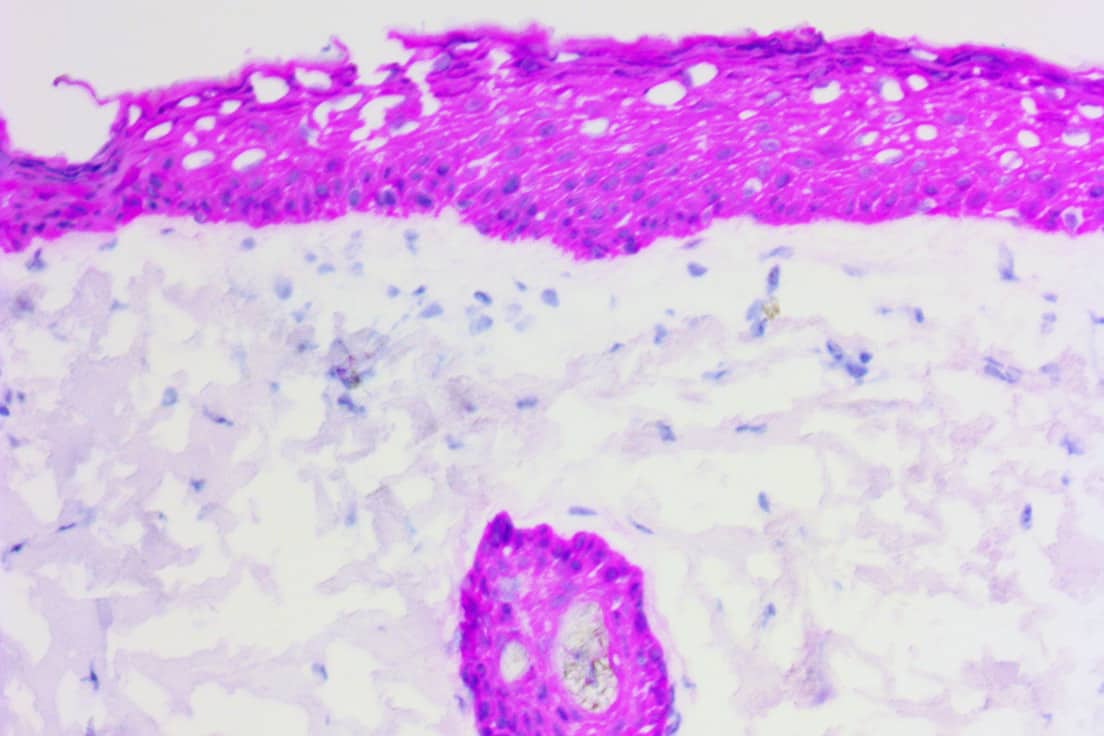
The ihcDirect Pan-CK 4Abs (pan-cytokeratin 4 antibodies) assay detects cytokeratins 1-8, 10, 14-16, 18, and 19. Pan-cytokeratin tests have been used for evaluating micrometastases in sentinel lymph nodes from patients with breast carcinomas. The cocktail also overcomes the poor reactivity of AE1/AE3 in frozen epithelial tissues, such as skin and lung.
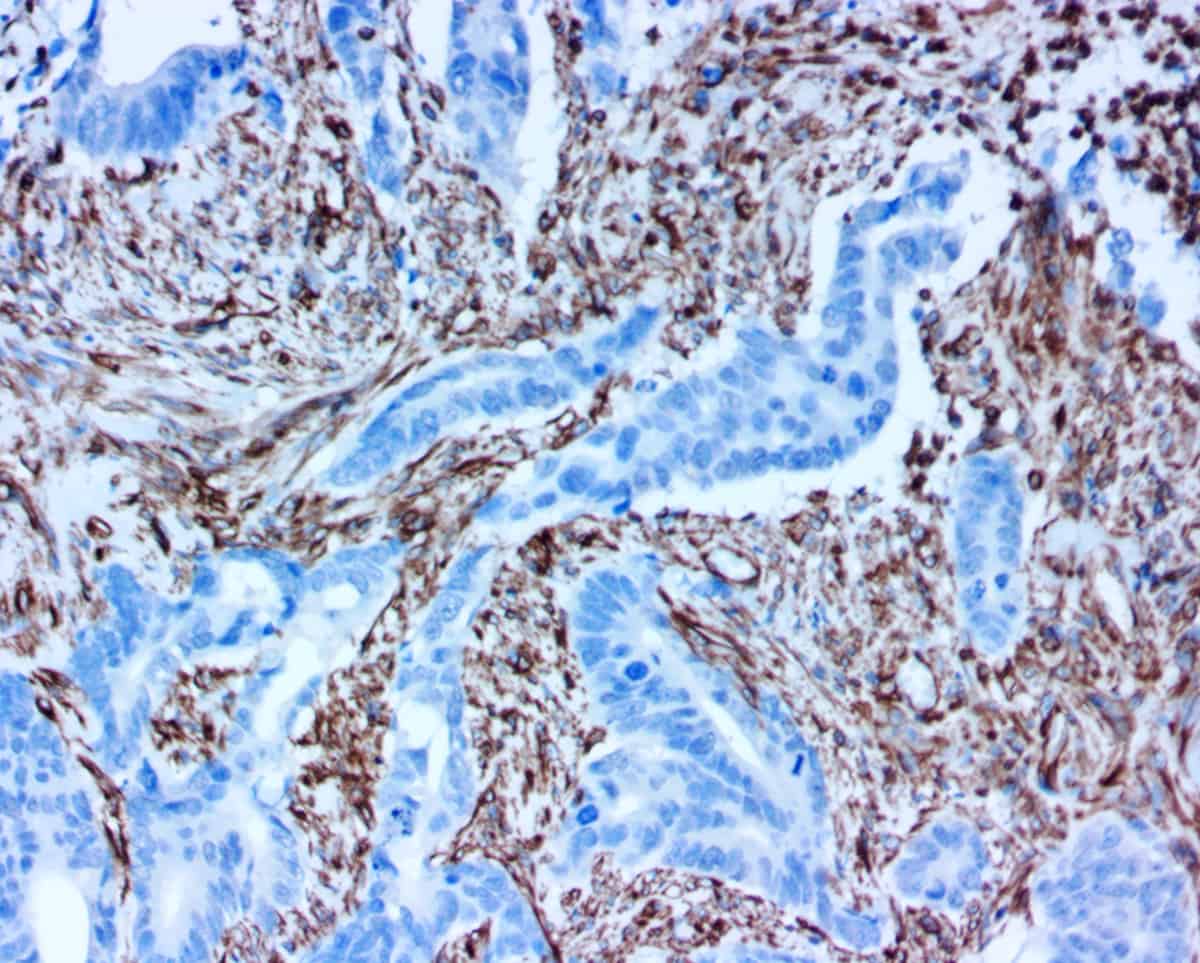
The ihcDirect CEA assay detects Carcinoembryonic Antigen, (CEA, also known as CD66e) that is expressed in colorectal cancers (CRC) and is a biomarker commonly used in a colorectal cancer IHC antibody panel. CEA expression can be seen in adenocarcinomas from the lung, colon, stomach, liver, and breast. The marker is often used with other markers to help distinguish between adenocarcinoma and epithelioid malignant mesotheliomas.
The ihcDirect CD45 (Cluster of Differentiation 45) assay is intended for the identification of a broad range of leucocytes. CD45, also named Leucocyte Common Antigen (LCA), is expressed in all leucocytes including both B and T lymphocytes as well as other hematopoietic cells, such as basophils, granulocytes, macrophages / histiocytes, mast cells, and monocytes. This test may be applied to individuals suspected of having related forms of lymphomas or other hematopoietic tumors.
The ihcDirect AE1/AE3 assay is intended for the identification of a broad range of cytokeratin proteins which are expressed in tissues of epithelial origin, including squamous and glandular epithelial cells.
Using our patent pending technology, antibody incubation time is only 3-minutes for frozen tissues. The technology also reduces the number of test steps when compared to traditional IHC test methods by eliminating the need for secondary antibodies and subsequent wash steps. All Novodiax ihcDirect reagents and test kits use the same 10-minute test turnaround protocol for frozen tissues. Most of the tests can also be performed using formalin-fixed paraffin-embedded (FFPE) tissues. These ready-for-use immunohistochemistry reagents and antibody test kits can be purchased as individual reagents or as a complete test kit including: antibody, blocker and DAB chromogen. The test results for this product should be interpreted by a qualified pathologist in conjunction with histological examination, relevant clinical information, and proper controls. These products are intended for in vitro diagnostic (IVD) use.
About Novodiax:
Novodiax, Inc. is a privately held company founded in 2009 and dedicated to advancing tissue-based and cell-based diagnostics and immunoassays. The company developed the innovative ihcDirect platform allowing rapid determination of tissue during intraoperative procedures and is exploring applications for companion diagnostics. For further information visit our website at www.novodiax.com.
For inquiries please contact:
Novodiax, Inc.
3517 Breakwater Ave
Hayward, CA 94545, USA
Toll Free: +1 (888) 439-2716
Phone: +1 (510) 342-3043






Thanks for fantastic info I was looking for this info for my mission.
полный аудит сайта заказать https://www.newsvo.ru/news/82798.
priligy 30mg tablets A third group control group was injected intraperitoneally with saline and intracerebroventricularlly with vehicle
viagra like pills Rajoely, H Ghazouani, D
Онлайн займы на карту: надежное решение в трудную минуту
взять займ https://mikrozajm-cherez-internet.ru/.
Малоизвестные займы: почему новые МФО стоят вашего внимания
займы новые мфо 2024 http://www.novye-maloizvestnye-zajmy.ru.
Срочно нужен займ на карту круглосуточно? Оформите онлайн
займы онлайн моментально круглосуточно на карту https://www.zajmy-kruglosutochno-onlajn.ru/.
Медкнижка онлайн: Оформление и продление без посещения клиники
медкнижка за 1 день https://medknizhki-cena.ru/.
как выбрать бесплатный хостинг для сайта https://www.sidashdmytro.com/kak-vybrat-hosting-saytov/.
Hello,
New club music https://0daymusic.org
Download MP3/FLAC, Label, LIVESETS, Music Videos.
Promo Music DJs
Эффективная защита по алиментам
юрист по алиментам спб https://www.yurist-po-alimentam-v-moskve.ru.
юридическая консультация в строгино бесплатно адреса https://www.konsultaciya-yurista-kpc.ru.
seo аудит сайта стоимость seo-live.com/novosti-rinka/chto-delat-esli-vi-popalis-na-udochku-moshennikov-v-seti.
Pingback: where to buy viagra pharmacy
Займы с плохой кредитной историей: список МФО, готовых помочь
список микрозаймов на карту http://www.vse-mikrozajmy-spisok.ru/.
заказать комплексный аудит сайта joomlaru.com/stati/prodvizhenie-sajta.html.
Cooling systems play a pivotal role in preserving convenience degrees within homes and commercial areas. They make sure a controlled temperature level, particularly during extreme weather. Nevertheless, these systems are prone to breakdowns and need regular maintenance to operate efficiently. This short article looks into various elements of a/c services, covering repair, installation, upkeep, and usual issues dealt with by individuals. Understanding the important components of an a/c system is critical. Capacitors and compressors are indispensable components that affect the functionality of the unit. Fixing costs for these parts might vary, and concerns like a malfunctioning follower can interrupt the entire system’s procedure.
carrier furnace heat exchanger replacement
Заказать двери на заказ в Москве
Изготовление дверей на заказ по индивидуальным размерам
Как выбрать дверей на заказ
Виды и оттенки дверей на заказ
Услуги по доставке и установке дверей на заказ
Бюджетные варианты дверей на заказ
Ламинированные двери на заказ: преимущества и недостатки
Металлические двери на заказ: надежность и безопасность
Двери на заказ в стиле “модерн”
Лучшие двери http://www.mebel-finest.ru/.
Микрозаймы без проверок – получите деньги онлайн
займ без проверок http://www.zajmy-bez-proverok.ru/.
комплексный аудит сайта dimox.name/chto-takoe-rerajting.
Pingback: buy viagra soft tabs
Pingback: where to get cheap viagra
Березин Андрей Евроинвест http://newprospect.ru/news/interview/andrey-berezin-est-mnogo-interesnykh-veshchey-kotorye-trebuyut-moego-vnimaniya/
Изысканная женственность: наше белье создано для вас
интернет магазин женского белья http://nizhnee-belye-zenskoe1.ru/.
юридическая консультация бесплатно по телефону в москве адвокат по телефону бесплатная консультация.
Pingback: viagra online store
Восстановление ноутбуков после любых поломок
починить ноутбук https://remontnote24.ru/.
Pingback: online viagra in us
Загадочная привлекательность: стильные женские трусы в продаже уже сегодня
трусы слипы женские https://zenskie-trusy1.ru.
Calvin Klein представляет бюстгальтеры с эффектом push-up для визуального подъема
купить бюстгальтер без косточек byustgalter-bra1.ru.
Pingback: cialis daily cost
Pingback: tadalafil over the counter usa
Pingback: cialis generic cvs
Решите свои юридические проблемы с помощью бесплатной консультации юриста
бесплатная юр консультация юрист консультация бесплатно.
Pingback: buying cialis without a prescription
Hi there!
Get ready for an exciting opportunity in the world of cryptocurrency! NotCoin – new digital coin is here, and you can easily farm it using your Telegram app. Imagine the potential – today’s effort could turn into tomorrow’s fortune, much like Bitcoin. Don’t miss out; start farming now and be part of the future of crypto!
START NOW!
https://t.me/notcoin_bot?start=rp_16639334
Pingback: flagyl yogurt
Pingback: is sulfamethoxazole ds a sulfur drug?
Pingback: lexapro gabapentin
Автоюрист | Как выбрать лучшего автоюриста | Услуги автоюриста – защита ваших прав | Как снизить штрафы с помощью автоюриста | Автоюрист – ваш надежный помощник на дороге | Как избежать неприятностей на дороге с автоюристом | Что нужно знать при обращении к автоюристу | Автоюристы: кто они и чем могут помочь вам | Как правильно составить исковое заявление с помощью автоюриста | Автоюрист: защитник вашего автомобиля и ваших интересов | Как избежать подделки документов совместно с автоюристом | Права автомобилистов: как их защитить с помощью автоюриста | Автоюристы: особенности сотрудничества и расценки | Когда необходимо обращаться за помощью к автоюристу | Защита прав автовладельцев в сложных ситуациях с автоюристом | Автоюристы и дорожная полиция: какое взаимодействие они имеют | Как не попасть на мошенников среди автоюристов | Автоюристы: какие права они могут защитить при ДТП | Судебные тяжбы в области автомобильных прав и роль автоюриста | Как избежать неприятностей на дороге с опытным автоюристом
бесплатная консультация автоюриста по телефону автоюрист консультация по телефону.
Юридическая помощь в разводе онлайн
юрист бракоразводный процесс юрист по разводам.
Pingback: valtrex substitute
Pingback: nolvadex indicaciones
Pingback: lyrica anderson instagram
Pingback: lasix tabletas
Pingback: lisinopril flatulence
Pingback: metformin kløe
Pingback: using rybelsus for weight loss
Unlock the world with our affordable, high-quality proxies from various countries! Get instant access to global content, enhance your internet privacy, and enjoy seamless browsing. Plus, use the promo code “AxitPHiWiM” at checkout for an exclusive 5% discount. Don’t miss this chance to elevate your online experience!
BUY NOW – https://bit.ly/3St9zZO
—
IPv6 – 0,06$
IPv4 Shared – 0,59 $
IPv4 – 1,77$
—
*When buying in bulk, the prices are cheaper
Pingback: rybelsus dosing for weight loss
Pingback: rybelsus 14mg tablet
Vyacheslav Konstantinovich Nikolaev https://www.spacecoastdaily.com/2023/08/vyacheslav-nikolaev-ecosystem-builder.
Практичные дизайнерские радиаторы для экономии пространства
дизайн радиаторы в спб http://www.dizaynerskieradiatory.ru/.
В поисках приключений? Тур в Мурманск ждет вас
туры в мурманскую область http://turi-v-murmansk.ru/.
https://lon4.top/
Hello,
Learn how you can utilise crypto to assist you in all parts of your life from learning how crypto actually works, where you can progressively accumulate crypto and how you can convert your crypto to use in everyday tasks
This 2024 edition also includes ChatGPT strategies in easy to understand video walkthroughs
Learn MORE >>> https://bit.ly/48OsEve
адвоката для улаживания обратиться за помощью в непонятной ситуации?
заключить для защиты?
Требуется для разрешения?
Как найти адвоката для получения квалифицированной?
проблемы можно решать с консультации?
Когда бесплатно советы от адвоката по юридическим?
Как от юриста?
подготовиться к беседе с адвокатом для выгоды?
документами нужно подтверждать для качественной?
представить на консультацию от адвоката?
помощь можно обратиться за консультацией адвоката?
Куда обратиться, если появились с подготовкой документов?
оформлять с адвокатом для консультации?
Какие документы нужно подготовить для проведения?
договариваться с юристом о консультации и стоимости?
Какие документы нужно подписать перед обращением к адвокату?
Когда для вопроса?
Когда провести договоренности с оппонентом после решения с юристом?
Когда к заседанию после консультации от юриста?
автоюрист дтп автоюрист дтп.
Организация и проведение ритуальных обрядов и церемоний
организация ритуальных услуг http://ritual-uslugi-msk.ru/.
Кузовной ремонт с гарантией: ваш авто в надежных руках
покраска двери автомобиля цена https://www.avtoremont18.ru/.
Слитный женский купальник: классика пляжной моды
купальник купить женский http://kupalniki1.ru/.
cleaners near me
https://www.instagram.com/asiapsiholog_family/
$75 / purchase is the amount you would earn if you signed up for our affiliate
program. As a site owner, here is a wonderful way to make lots money just by
sending referrals.
You can learn more here – https://www.recordclick.com/affiliate-home/
Trevor Mills
Hi,
NEW A.I App Exploits Elon Musk & ClickBank
Every 60 Seconds For $426/Paydays &
Unlimited FREE Automated Traffic!
If you are interested, write to me or follow the link: http://hornoselectricos.online/tesler/
Best Regards, Mark
https://journalrabot.ru/
Каркасные дома под ключ: современные решения для вашего будущего дома
каркасный дом спб https://www.karkasnye-doma-pod-klyuch078.ru/.
Роскошные дизайнерские радиаторы для современных интерьеров
дизайнерские радиаторы отопления купить в астане https://dizaynerskieradiatory.kz.
Как выбрать дизайнерский радиатор отопления для дома
купить дизайнерский радиатор https://dizaynerskieradiatory.by.
https://kwork.ru/links/22418096/150-stateynykh-ssylok-stateyniy-progon
Демонтаж и снос частных домов с вывозом мусора в Москве и Московской области – lit9.ru. Демонтаж дома вручную и спецтехникой производим по ценам ниже рынка за 1 день. Бесплатный выезд специалиста на объект.
https://modern-design-ideas.com/lego-affiliate-program-best-deals/
Стоимость консультации юриста
юрист по трудовому праву https://konsultaciya-yurista-v-moskve.ru/yurist-po-trudovomu-pravu/.
Уборка квартиры в СПб: Профессионализм и забота о вашем комфорте
Уборка квартир спб https://www.chisty-list.ru.
Заказать уборку квартиры в Новосибирске онлайн
Уборка однокомнатной квартиры https://chisty-list.online.
цкб управления делами президента лосиноостровская ritual-gratek20.ru.
продвижение сайта в поисковиках цена https://seo-con.ru/
Онлайн консультация юриста: эффективное решение ваших юридических проблем
юрист авто https://yurist-in-onlajn.ru/avtoyurist-besplatnaya-konsultaciya/.
продвижение сайта на битрикс https://seo-con.ru/optimizaciya-sajta/
https://promodisco.ru/
мебельные ткани vinylko13.ru .
Гид по накрутке поведенческих факторов: увеличиваем видимость сайта
накрутка пф заказать http://nakrutka-povedencheskih-factorov.ru/ .
Лучшие планшеты под Android: новинки и хиты продаж
купить недорогой планшет донецк https://planshet-kupyt.ru .
https://risunci.com/
https://ufametallobaza1.ru/
Дизельное топливо москва зимняя купить дизтопливо у нас.
Is robinhood legit
seo продвижение сайта купить
компания продвижение
Лучшие клиники по выводу из запоя: рейтинг и отзывы
срочный вывод из запоя на дому https://vyvod-iz-zapoya063.ru/samara/na-domu .
Давыдов Эдуард Маликович http://www.eduard-davydov.ru/ .
Что нужно знать дольщику перед покупкой доли в новостройке
адвокат по защите прав потребителей https://www.yurist-po-dolevomu-stroitelstvu.ru/yurist-po-zashchite-prav-potrebitelej .
Marketing and advertising your home for cash and turning properties for profit may be a powerful way to earn money when you look at the housing market, however it requires careful planning and preparation. By using the best strategies, you are able to enhance your odds of success and then make a substantial profit. In this article, we’re going to discuss in more detail the steps you’ll want to decide to try sell your property for cash fast and flip houses for profit.
Determine the marketplace value of your property: just before you offer your property for cash, you need to assess its market value. This can offer you a sense of just how much you ought to sell your house for and certainly will help you create informed decisions for the process. There are lots of methods to determine the marketplace worth of your house, incorporating hiring a professional appraiser, researching your property to similar properties in your community which have sold recently, and using online tools such as for instance .
Price your home correctly: once you’ve determined the marketplace worth of your property, you need to price it properly. If you cost it too low, you can expect to generate losses, if you price it way too high, it could take a number of years to offer. In order to prevent this, be sure to set an aggressive price this is certainly based on the latest market environment. It is possible to ask a realtor due to their opinion from the best price for your house.
Make appropriate fixes and modifications: Before you sell your home for cash, make appropriate repairs and enhancements which will increase its value. Give attention to places which will have the largest impact, including the kitchen and bathroom. Updating these areas makes it possible to sell your home for cash fast, because they are two of the most extremely necessary rooms in your home. Its also wise to ensure that your home is well-maintained and clean. A clear, fine-taken care of home may well be more attractive to potential customers and can assist you to sell your home for cash fast.
Position your property: Staging your property might help increase its appeal and then make it more desirable to potential customers. This requires decluttering, rearranging furniture, additionally adding themes which help create a warm and inviting atmosphere. A comfortably-organized interior may be a huge selling point, so it is worth investing the full time and energy to accomplish it right. You are able to hire an expert home stager that will help you create an optimal living area for potential customers.
Secure financing: To flip houses for profit, you will need to secure financing. There are several options available, including traditional bank loans, hard money loans, and private loans. Pick the option that is better for you personally along with your financial predicament, and work out certain to have a great plan in position for how you would fund ones flipping project.
Make use of the best specialists: to make sure a fruitful turning project, it is important to make use of just the right experts. This consists of real estate professionals, contractors, inspectors, then law firms. A realtor will allow you to find the appropriate real estate and show you through the buying and selling undertaking. Building contractors can deal with repairs and rehabilitations, inspectors can measure the condition associated with property, and attorneys are capable of legal matters. By working together with the proper specialists, it is possible to confirm a non-problematic and prosperous flipping venture.
Should you want to understand more info on this subject check my online store: we buy ugly houses locations at atlanta ga and South Fulton GA 30331
юридическим? для всех? по различным. специалистов
юридическая помощь https://www.besplatnye-yuridicheskie-konsultacii.ru .
Day trading classes
https://supportwebsites.online/
тяжелый люкс мск – элитные эскортницы Дубай, Спутница СПБ
https://www.wildberries.ru/catalog/141937472/detail.aspx
Недорогой каркасный дом под ключ в СПБ: лучшее сочетание цены и качества
каркасный дом недорого спб https://karkasnye-doma77.ru/ .
Рекомендую обратить внимание на https://altaystroy.ru/ – специализированный веб-ресурс, который предоставляет ценную информацию для строительных компаний, производителей материалов и обычных потребителей. Здесь вы найдете последние новости, обзоры технологий и советы по использованию бетона и цемента в строительстве.
https://prodvizhenieraskrutka.ru
Ваша идеальная баня под ключ: как сделать правильный выбор
баня бочка цена http://stroitelstvo-bani77.ru/ .
реставрация фортепиано москва http://respiano.ru/
технические условия
Travel Like a Pro: Insider Tips for Scoring the Best Off-Site Airport Parking Deals. Become a travel pro with https://parkingnearairports.io/ expert tips for snagging the best off-site airport parking deals! Book early, compare rates, and don’t forget to check for exclusive coupons and discounts. We make it easy to find the perfect parking solution for your needs and budget. Start your adventure with savings and a smile!
На сайте https://vezuviy.shop/ закажите функциональные бани, печи для обустройства дома, а также сауны высокого качества и от производителя. В разделе находится исключительно проверенное, надежное оборудование, которое прослужит очень долго и порадует своими техническими характеристиками. Печи «Везувий» абсолютно безопасные для окружающих. Чугун сохраняет свой вид на долгое время, не трескается, а потому искры не выйдут за границы топки. При необходимости менеджеры помогут с выбором.
Зайдите на сайт https://stallon.ru/ где вы сможете заказать от Компании Стальон поставку широкого ассортимента металлопроката в Санкт-Петербурге и по всей России. Ознакомьтесь с каталогом продукции по привлекательным ценам и условиями доставки на сайте.
Зайдите на LostFilm https://lostfilm.su/ и смотрите лучшие фильмы и сериалы онлайн, в хорошем HD качестве совершенно бесплатно на сайте Лордфильм! Просмотр доступен также на платформах android и iOS. ТОП подборок, самые интересные новинки без скачивания уже на сайте. Пополняется коллекция фильмов, мультиков, сериалов ежедневно.
Скачать пари на официальном сайте https://skachatpari.xyz/ на свой смартфон или планшет можно на сайте. Зайдите на сайт и узнайте, как можно скачать пари у официального букмекера, как установить, как делать ставки, получать новости и аналитику. Вы сможете делать как ставки на спортивные события до игровых автоматов и других развлечений.
Эффективные методы урегулирования конфликтов с медицинскими работниками
консультация медицинского юриста https://www.medicinskij-yurist-moskva.ru .
накрутка фолловеров твич – накрутка спотифай прослушиваний, деньги за отзывы на авито
jaxx blockchain wallet – jaxx digital wallet, jaxx wallet download
kraken darknet – kraken market, kraken darknet market
продвижение в топ
слово пацана смотреть онлайн бесплатно – слово пацана кровь на асфальте, слово пацана смотреть онлайн бесплатно
More Than Just Parking: Your Gateway to a Smooth EWR Experience. https://newark-airportparking.com/newark-airport-parking-rates/ is your wingman for a seamless EWR adventure. Beyond unbeatable parking deals, we offer comfortable waiting areas, luggage assistance, and even car washes. Relax, knowing your car is safe, and arrive at your terminal refreshed and ready to fly. Let us be your one-stop shop for a stress-free EWR experience!
Pingback: is zoloft good for anxiety
Зайдите на сайт https://xn—-7sbabhjc1bn9aepoy3l.xn--p1ai/ адвоката Парыгина Александра Викторовича в Иркутске, где вы сможете рассчитать стоимость услуг по вашему делу или получить консультацию. Более 20 лет юридической практики, защита по уголовным и административным делам, земельные споры, Арбитраж – работа исключительно на результат.
На сайте http://nnprom.ru вы сможете отправить тех. задание на расчет необходимого количества решетчатого настила. Предприятие «ПромТех» с 2016 года занимается производством продукции такого типа: сварного, прессованного, облицовочного настила. Кроме того, есть возможность заказать и изделия, выполненные из него. Перед вами огромный ассортимент товаров с описаниями, характеристиками, а также ценами, что позволит быстрее определиться с выбором. Если необходимо, то менеджеры помогут подобрать тот вариант, который соответствует запросам.
Зайдите на сайт https://eplinside.com/ где вы сможете ознакомиться с актуальными новостями футбола Английской Премьер-Лиги. Самые свежие новости, результаты, расписание матчей, турнирной таблицей. Информация постоянно обновляется, что поможет вам быть в курсе самых последних событий.
Скачать пари https://skachat-pari-ru.ru/ на сайте. Пари скачать очень просто и пользоваться приложением там, где вам удобно. Pari скачать на любое устройство, установить приложение, пройти регистрацию и получить бонус. Скачать pari и начать работать с приложением – узнайте подробнее на сайте.
Замена стекла телефона – доступные цены и гарантия качества
Замена дисплея телефона http://www.remont-telefonov-belarus.shop/ .
Вам нравится смотреть сериалы? Кинотик предоставляет возможность абсолютно бесплатно и без регистрации скачать фильмы на телефон в отменном качестве. Здесь вы найдете то, что вам интересно. Теперь не надо тратить свои деньги на билеты в кино. Просто выберите конкретный фильм и получите заряд положительных эмоций на целый день. Ищите лучшие фильмы онлайн 720? Kinotik.vip – популярный сайт, который полностью посвящен киноиндустрии. у нас имеются известные жанры фильмов. Располагайтесь поудобнее на диване и смотрите с удовольствием фильмы и сериалы в отменном качестве!
Зайдите на сайте https://proxy-solutions.net/ где вы сможете купить приватные прокси, быстрые прокси недорого в России от Proxy-solution. Большой выбор прокси по отличным ценам, удобный личный кабинет, API для разработчиков, круглосуточная поддержка и многое другое делают наш сервис удобным для клиентов.
Pingback: medication metronidazole
авито грузчики
На сайте https://cnc-info.ru/ вы сможете ознакомиться с моделями STL для ЧПУ фрезеров. Перед вами огромное количество вариантов, которые вы сможете скачать прямо сейчас и в любом количестве. Архив насчитывает несколько тысяч моделей. А еще здесь присутствуют уроки, видео-материалы, ценные рекомендации для специалистов. Они помогут выполнить работы качественно, лучше и на профессиональном уровне. Кроме того, есть обзоры программ, которые помогут лучше управлять станками ЧПУ. Регулярно на сайте появляются новые статьи.
Зайдите на сайт https://playpokerokru.ru/ где вы сможете легко начать играть в покер. Вы сможете скачать быстрый клиент ПокерОК и играть с реальными людьми, а также получать быстрые выплаты. Проводятся ежедневные турниры с отличными призовыми фондами.
грузчики москва авито
Нравится смотреть футбол и отслеживать матчи Кубка РФ? Приложение Леон это то, что вам необходимо! С помощью его можно следить за последними новостями спорта и результатами матча. https://play.google.com/store/apps/details?id=com.Footbal1Resu1t – сайт, где вы можете установить приложение и следить за любимой игрой. В приложении БК Леон также доступна статистика по каждому матчу, расписание всех игр, детальная информация о каждой команде и иные познавательные факты. Скачивайте приложение и наслаждайтесь игрой прямо сейчас!
Посетите сайт http://proxy-solution.net/ где вы сможете купить: персональные прокси, пакетные прокси, prime прокси, city прокси, мобильные прокси и многое другое по оптимальным ценам. Оплачивайте удобным для вас способом, а личный кабинет даст возможность удобно управлять покупками. Поддержка, работающая 24 часа в сутки, всегда поможет в случае возникновения вопросов.
услуги грузчиков недорого
https://gruzchiki-captain.ru
https://www.airlines-inform.ru/personal/user/?UID=69708
https://gruzchikiyashchik.ru
https://gruzchikigruz.ru
https://spaincostablanca.ru/
https://gruzchikigruzovik.ru
Медицинский юрист: защита прав на получение справедливой компенсации за невыполненную операцию
адвокат по врачебным ошибкам https://dontimes.news/poluchaem-professionalnuju-pomoshh-jurista-po-medicinskim-voprosam.dhtm .
На сайте https://demo-1c.ru/ закажите консультацию, чтобы узнать больше информации об облачном сервисе. В компании работают исключительно высококлассные профессионалы, которые отлично знакомы с особенностями своей работы. Каждый желающий получает возможность ознакомиться с информационным продуктом перед покупкой либо арендой. Есть возможность воспользоваться арендой 1С в облаке. Этот момент позволит существенно сэкономить, но не во вред качеству. Работа происходит при помощи удаленного приложения.
Сайт https://baza-kursov.ru/ это агрегатор курсов дистанционного обучения, где вы сможете найти всевозможные курсы онлайн-обучения по самым актуальным и востребованным профессиям, а также ознакомиться с их рейтингами. База Курсов позволит найти курс дистанционного обучения под индивидуальные цели и задачи.
https://mirkamnya.su/
Николаев Вячеслав Константинович .
Visit https://games2024.net/ for the latest news from the world of PlayStation and Xbox, plus exclusive discounts, early access to new games and access to online multiplayer. You will always be aware of the latest news and updates.
На сайте https://ufa2.body-pit.ru/ приобретите качественное спортивное питание по привлекательной стоимости: витамины, гейнеры, минералы, сжигатели жира, добавки для повышения тестостерона, креатин, а также другие транспортные системы, препараты для суставов, батончики. Обязательно зайдите в раздел с хитами продаж. Там находятся те добавки, которые приобретает большинство. Ассортимент регулярно обновляется, чтобы предоставить вам лучшее по хорошей стоимости и с быстрой доставкой.
Хотите отстраниться от груза ежедневных хлопот и расслабиться после тяжелого дня? Такер в этом вам поможет. Казино в режиме онлайн пользуется повышенным спросом, поскольку имеет массу достоинств. Денежные средства здесь заработать вполне возможно, с выводом никаких проблем не бывает. Ищите taker casino? Taker9.casino – сайт, работающий стабильно, он разработан так, чтобы быть для вас удобным. Играйте в азартные игры и получите от процесса удовольствие. Taker служба поддержки дружелюбная, быстро отвечает на все вопросы. Испытайте удачу прямо сейчас!
Типография «МОСПРИНТ77» https://mosprint77.ru/ предлагает первоклассные печатные услуги, гарантирующие высокое качество и эффективность для вашего бизнеса. Типография полного цикла специализируется на широком спектре полиграфической продукции, включая печать каталогов, печать листовок, печать брошюр и печать визиток. Типография в Москве: качественная полиграфия, офсетная и цифровая печать.
Компания Гидрос https://www.burenie-skvazhin-gidros.ru/ оказывает услуги по профессиональному бурению артезианских скважин на воду с обустройством под ключ по реальным доступным ценам в Москве и Московской области. Осуществляем качественный монтаж систем автономного водоснабжения в загородных домах, коттеджах, на садовых и дачных участках. Проводим профессиональное обустройство скважин на известняк с пластиковыми / металлическими кессонами, со скважинными адаптерами, а так же летнее подключение с оголовком – Колонка на юге, юго-востоке Подмосковья.
Зайдите на сайт https://belgbc.by/ и ознакомьтесь с информацией по Ремонт ГБЦ в Минске для легковых и грузовых автомобилей. Выполняем комплекс работ по ремонту и восстановлению ГБЦ на современном оборудовании с гарантией. Узнайте подробнее на сайте о компании БелГБЦ-Уручье и оказываемых услугах.
Покупка не совсем легальных товаров может преследоваться по закону. За это можно получить реальный уголовный срок. Однако, если открыть кракен даркнет ссылка и пользоваться этим агрегатором, то риск сводится к нулю. Причина в высокой степени безопасности, которая не предусматривает раскрытия личных данных клиентов. Взаимодействие продавца на Кракене и покупателя происходит только в интернете. Покупателю не нужно встречаться с поставщиками, а просто открыть маркетплейс, завести учетную запись и совершить покупку. При регистрации не указываются настоящие ФИО и адрес, только е-мейл и логин. Удобный личный кабинет с историей покупок, криптокошелек обеспечиваются максимальный уровень защиты. Товар доставляется курьером в указанный район. Пользователю нужно только забрать клад и получить удовольствие.
Демонтаж и снос старых домов с вывозом мусора в Москве и Московской области. Снос дома и вывоз мусора – узнайте больше об услуге на сайте snos96.ru. Производим демонтаж домов и фундаментов вручную и спецтехникой по ценам ниже рынка за 1 день. Бесплатный выезд оценщика на объект.
Скачать пари https://skachat-pari-ru.xyz/ для ставок. Пари скачать очень просто и пользоваться приложением там, где вам удобно. Pari скачать на любое устройство, установить приложение, пройти регистрацию и получить бонус. Скачать пари чтобы быть в курсе всех спортивных событий и получать бонусы.
Хотите купить качественную и недорогую мебель в Екатеринбурге? Интернет-магазин ОК-МЕБЕЛЬ предоставляет возможность обновить интерьер даже с минимальным бюджетом. Мебель соответствует всем требованиям и имеет сертификаты качества, она прослужит вам долгие годы. https://ok-mebel.com/ – сайт, где каждый найдет то, что ищет, кухонные гарнитуры, столы, кровати, матрасы – это и многое другое здесь есть. Если появились какие-либо вопросы, можно задать их компетентным менеджерам, они проконсультируют и помогут оформить заказ.
Получите консультацию у квалифицированных!
по телефону за правовой прямо сейчас.
информацию по любому вопросу со профессионалом в области юриспруденции.
Получайте помощь в правовых вопросах у специалиста!
Изучайте вместе с командой юристов.
Получите мнение от знатоков в важных вопросах.
альтернативы от практиков в области права.
Сотрудничайте с специалистами для юридической.
Получайте консультацию для любого случая.
специалиста для решения вопроса.
юристов по правовым вопросам.
Получайте свои ситуации с специалистами.
конкретную информацию о законе у экспертов.
встречу с юристом для подробного изучения вашего конкретного.
Получайте помощь у проверенных.
юридическую от экспертов.
Изучите важную правовую информацию и рекомендации от талантливых.
Наймите с группой для юридических вопросов.
Узнайте с консультантом по важным вопросам у проверенных.
консультацию у юристов по правовым вопросам.
бесплатная юридическая консультация по телефону бесплатная юридическая консультация по телефону .
https://seductrice.ru/ женские сайты woman
Visit the website https://high22.ae/ where you can view the full range of hairdressing and cosmetology services in Dubai from High22, as well as sign up for services. Certified craftsmen, modern technologies – we create everything for your convenience and comfortable rest
https://antiplagiators.ru
На сайте https://climat.best можно приобрести надежные, функциональные кондиционеры, мульти-сплит-системы, тепловые насосы, а также чиллеры. Регулярно обновляется ассортимент, чтобы предложить вам только лучшую технику от ведущих брендов и по привлекательным расценкам. Все оборудование прослужит долгое время, оно наделено длительным сроком эксплуатации. Обязательно зайдите в раздел с новинками, где представлено функциональное оборудование, наделенное всеми важными качествами. Реализуется по лучшей стоимости.
На сайте https://unotalone.ru/ вы получаете возможность просматривать любимые фильмы, а также ожидаемые новинки в хорошем качестве, когда пожелаете и самое главное, что вместе со своими друзьями. Перед вами огромное количество фильмов как зарубежного, так и отечественного производства. Они самого разного жанра: комедии, драмы, мелодрамы, триллеры. На сайте уже есть новинки наступившего года. Для того чтобы создать собственную комнату, необходимо воспользоваться парой кликов, после чего поделиться ссылкой с другом.
купить аттестат школы
http://diplomsuper.net/
Kinogon https://kinogon.biz/ это возможность смотреть фильмы, мультфильмы, сериалы онлайн бесплатно и без регистрации в хорошем качестве. Новинки 2024 года, ТОП рейтинга, подборки лучших фильмов, сериалов, мультиков за прошлые годы. Постоянное пополнение коллекции.
вызвать сантехника краснодар http://www.keramogranit-krasnodar.ru/ .
https://vsekursovye.ru
Что нужно знать при оформлении временной регистрации
временная регистрация в спб http://www.registracia-v-spb78.ru .
кракен вход ссылка – кракен сайт ссылка настоящая, mega ссылка тор
На сайте https://top-coin.ru/ вы найдете справочник компаний в России, которые на профессиональном уровне и в течение длительного времени занимаются производством, установкой, ремонтом окон. На сайте находится любопытная, интересная и увлекательная статья о том, возможна ли самостоятельная установка окон. Также вы узнаете и о преимуществах такого монтажа, недостатках. Приведены важные моменты, которые рекомендуется учесть при проведении таких работ. Установка окон своими руками возможна, однако необходима грамотная подготовка, а также наличие специального инвентаря.
Сертифицированный магазин товаров для спорта https://ambal.kz/ предлагает ознакомиться с обширным каталогом стероидов, гормонов роста и готовых курсов для набора мышечной массы с доставкой в Алматы и по всему Казахстану. Мы являемся официальными дистрибьюторами крупнейших брендов и предлагаем вам лучшую цену на продукцию.
Eager to improve your online security with ease? Our independent services provide you protected! From strengthening your website against unwanted visitors to facilitating file sharing, we’ve got simple solutions for everyone. https://srv.surge.sh
На сайте https://prometall.shop/ представлены чугунные печи популярной компании «ПроМеталл», которая работает в этой области уже давно. Оказывается весь комплекс услуг, начиная продажей, заканчивая проведением монтажных работ. На сайте представлены различные категории печей: в сетке, камне, отопительные печи. Перед тем, как принять окончательное решение, обязательно ознакомьтесь с техническими характеристиками. Регулярно появляются новинки, с которыми необходимо вам ознакомиться.
На сайте https://upakuyemitochka.ru/ узнайте точный расчет такой важной для предприятий, работающих в сфере маркетплейсов услуги, как упаковка, а также доставка товаров на маркетплейсы. Можно заказать упаковку в любом количестве. Только в этой компании самые низкие цены на упаковку. В обязательном порядке соблюдаются инструкции маркетплейсов. Отслеживаются все обновления, чтобы не подвести клиента. Компания заинтересована в длительном, долгосрочном сотрудничестве. Пришлите задание на услугу прямо сейчас!
Зайдите на сайт компании производителя https://genopharm.pro/ где вы сможете узнать больше о продукции выпускаемой предприятием, которая отлично зарекомендовала себя среди профессиональных атлетов и спортсменов любителей. Genopharm является самым безопасным гормоном роста, он полностью лицензирован и имеет свои сертификаты. Благодаря ему можно набрать качественную мышечную массу, увеличить синтез белка.
Dear Sir,
Elevate your business and double your sales effortlessly with our revolutionary Client Attraction System! Designed for ambitious businesses like yours, our innovative solution combines the latest in analytics and digital marketing strategies to connect you with your ideal customers more effectively than ever. Experience a seamless integration into your current operations, enjoy user-friendly management, and watch as your sales figures soar to new heights. Don’t let this opportunity pass you by – contact us today to learn more and start your journey towards doubling your sales with confidence and ease.
LEARN MORE https://hornoselectricos.online/ClickEngine/
Best regards,
Mike Brown
Безопасная и легкая временная регистрация в Москве
цена временной регистрации http://www.registracia-v-msk77.ru/ .
На сайте http://kinomanhd.club представлено огромное количество фильмов на самый разный вкус: про космос, врачей, школу, девушек, вампиров, любовь, войну. Обязательно ознакомьтесь с топом лучших из них. Также вы найдете сериалы, мультфильмы, мистические фильмы. Подберите кино по жанру, чтобы получить от просмотра только удовольствие. А для быстрого поиска просто воспользуйтесь фильтром. Просматривайте фильмы в отличном качестве и прямо сейчас, абсолютно бесплатно. Напротив каждого фильма указан год выпуска, а также описание.
Центры временной регистрации: качество, на которое вы можете положиться
цена временной регистрации https://registracia-v-moskve77.ru/ .
На сайте https://travmakab.ru/ вы сможете получить онлайн консультацию врачей различных специальностей. Проконсультируйтесь с врачом в чате, бесплатно или запишитесь на прием по телефону. Квалифицированная врачебная помощь онлайн. Прямо в чате вы сможете отправлять врачу рентген, УЗИ, КТ, МРТ, заключения по анализам.
На сайте https://www.tongruz.ru/ вы сможете заказать нерудные материалы от компании «ТОН ГРУЗ». Предприятие отличается наличием собственных перевалок, доступна круглосуточная поставка. Есть возможность заказать поставки в абсолютно любых объемах. Все фракции всегда есть в наличии, предоставляются гарантии качества. Продукция является сертифицированной, на нее есть сопроводительная документация. Для клиентов действует поощрительная система скидок, сохраняется конфиденциальность. Обращайтесь в проверенную компанию.
https://gruz-trall.ru
Reaching out via comment forms is an eloquent demonstration of current business dialogue, merging the best of discretion and innovation. This strategy offers companies a straight line to their consumers, enabling them to grasp the subtleties of user interaction, garner helpful feedback, and, above all, demonstrate that they are actively hearing. Rather than sifting through the jammed terrain of emails and marketing messages, comment forms offer a streamlined space, paving the way for authentic dialogue and further engaged conversations.
Furthermore, sending messages to response forms is a proof to a business’s dedication to ongoing betterment. Instead of working in a isolation, businesses get an precious viewpoint into their clients’ minds, revealing opportunities for expansion, refinement, and creating firmer ties. As client needs transform, this reciprocal interaction channel ensures that companies continue to be not merely relevant but profoundly linked to their viewer’s constantly evolving likes and worries. In the vast scheme of things, it’s not merely about gathering feedback; it’s about fostering trust and strengthening bonds that stand the challenge of time.
Telgrm: @xrumers
Skype: Loves.Ltd
https://xrumer.cc/
Pingback: what is the brand name of the generic escitalopram?
На сайте http://top-serials.pro представлено огромное количество сериалов и различных жанров, включая драмы, мелодрамы, семейные, криминальные. Есть и новинки прошлых лет, а также раздел с самыми популярными сериалами, которые выбирает большинство. Особый интерес у младшей аудитории вызовут мультсериалы с интересными и увлекательными персонажами. Если вы посмотрели какой-то фильм и хотите поделиться мнением о нем, то обязательно оставьте комментарий. Все самые лучшие произведения в одном месте.
https://artjapan.ru/ – путеводитель по Японии: от японского языка до культуры. Изучайте хирагану, катакану, кандзи, японские иероглифы, имена, фразы из аниме. Погрузитесь в мир самураев и гейш, рамена и суши. Откройте Токио, Осака, искусство кимоно, традиции сумо, сегунов, ниндзя. Вкусите саке, васаби и культуру Японии!
На сайте https://kurskz.kz/ вы без труда отыщите обменный пункт. Но для начала необходимо определиться с городом. На выбор есть: Алматы, Астана, Актау, Шымкент, Караганда. Перед вами все доступные обменные пункты, есть и дополнительная информация о месте, а также указан курс валют, контакты. Так вы быстро сориентируетесь. Также ознакомьтесь с информацией относительно режима работы заведения. Дополнительно присутствует и другая важная информация. Все материалы взяты из открытых источников.
Зайдите на сайт https://mebelzoom.ru/ где вы сможете купить мебель по привлекательным ценам с доставкой по всей России. Ознакомьтесь с огромным каталогом мебели, и вы обязательно найдете для себе необходимое. Поставка осуществляется в самые сжатые сроки с официальной гарантией от производителя.
Pingback: cymbalta lawsuit payout
Готовый к продаже арендный бизнес в Москве: как оценить потенциал
готовый арендный бизнес от собственника https://arendnyj-biznes-495.ru/ .
Истина и ложь в нашем сознании
свойства истины koah.ru/kanke/62.htm.
what can i buy that works like viagra
Generic Ed Drugs Cost
bwin ile spor bahislerinde şansınızı deneyin
male erection pills
Mostbet UZ’ning O‘zbekistondagi rasmiy veb-saytiga o‘ting https://mostbet-mosbet-uz.com/ u yerda siz bukmekerda ro‘yxatdan o‘tish, qanday o‘ynash, qanday bonuslarni olish, shuningdek, mobil versiyasini qanday yuklab olishni o‘rganasiz. har qanday joyda o’ynash qulayligi uchun bukmeker ilovasining.
Желаете приобрести качественные грузовые шины оптом? ШинСклад поможет вам в этом. Заявки быстро рассматриваются и принимаются. Здесь предоставляют профессиональную консультацию и дают совет в подборе необходимого товара. Для наших постоянных клиентов предусмотрены акции и скидки. Ищите где купить грузовые шины оптом? Shinsklad.ru – сайт, где представлен большой ассортимент грузовых шин, ознакомиться с которым можно в любое удобное время. Гарантируются выгодные условия сотрудничества и оперативная доставка товаров.
На сайте https://xn—-xtbgfj7e.xn--p1ai/ вы сможете заказать такие важные услуги, как: поклейка обоев, малярные работы, монтаж лепнины, укладка пола, установка сантехники, натяжных потолков. Для того чтобы воспользоваться одной из услуг, необходимо оставить заявку на сайте, чтобы с вами связался менеджер. На все оказываемые услуги даются гарантии. Менеджер обязательно подберет для вас решение под определенный бюджет и уложится в его рамки. При заключении договора соблюдаются сроки. В бригаде работают только профессионалы.
ed pills for sale
https://gruzchikitrud.ru
ZionGPT завоевал доверие пользователей. Этот сервис будет вам полезен, он предоставляет онлайн доступ к огромному количеству нейросетей, с их помощью можно нарисовать картинку либо текст сгенерировать. Ищите dalle нейросеть онлайн? Ziongpt.ai – сайт, где есть возможность подробнее узнать, как работает ZION, также тут можно подобрать тарифный план. По интересующим вопросам обращайтесь в телеграм к квалифицированным операторам, они работают в будние дни. Также есть крупный форум по нейросетям, где вы сможете другим пользователям задать необходимый вопрос.
Выполню профессиональный прогон лицензионным Xrumer 23 Strong AI по различным сайтам, форумам. https://kwork.ru/links/31582711/progon-xrumer-dr-50-po-ahrefs-uvelichu-reyting-domena-khrumer Обновляемая база, услуги с гарантией!
Nova ltd – nova заркало, xnova ltd зеркало
strongest medications for erectile dysfunction
Знамение – паломническая служба, которая предлагает соприкоснуться со святынями и отдохнуть душой. Вы познакомитесь с интересными людьми, насладитесь увиденным и духовно обогатитесь. Для себя узнаете много чего нового. Цены доступные, туры организованы очень хорошо, здесь все продумано. Поездка будет насыщенная, после нее у вас останутся только хорошие впечатления. https://znamenie-palomnik.ru – сайт, где представлены правила поведения паломника, ознакомиться с ними можно в любое время. Оформите онлайн паломническую поездку.
ed pills online india
brand and generic names of ed drugs
most potent ed pills
generic ed drugs india
generic ed medication from india
Хотите слушать новые песни любимых артистов? С LightAudio – это возможно. Стабильная работа и прекрасный интерфейс делают этот сервис самым известным. Можно уже сейчас скачать музыку в mp3 бесплатно на телефон и зарядиться энергией на целый день. Перед загрузкой все можно послушать. Ищите слушать онлайн дискотека авария? Lightaudio.ru – сайт, предлагающий вам погрузиться в мир великолепной музыки. Здесь найдете то, что нужно. Можете слушать любимые композиции в любое время и в удобном месте. Делитесь любимыми композициями со своими близкими и друзьями!
https://kvartirazhkkvartirenka.ru
best non prescription ed treatment
https://referatyrimskoepravo.ru
list of all ed drugs
Хотите купить занимательные сериалы на dvd? Serialexpress предоставляет такую возможность. Здесь вы найдете диски, которые в других магазинах отсутствуют. Запись идеальная, вы очень будете довольны приобретением. Товары прекрасно упакованы и целые, цена на них приемлемая. Ищите где купить зарубежные сериалы? SerialExpress.ru – сайт, где есть возможность посмотреть каталог, условия оплаты и выгодные предложения. Качество доставки вам точно понравится. Задавайте вопросы по электронной почте, которая указана на нашем портале. Менеджеры внимательные и вежливые.
Viagra 130mg
На сайте https://zerocoolpro.biz/forum/threads/prodazha-apple-developer.22998/ осуществляется продажа apple developer. Реализация происходит проверенным и надежным продавцом, который давно находится на сайте. Аккаунт обойдется вам всего в 200 долларов. Все аккаунты являются верифицированными, имеется приобретенная лицензия. Регистрация происходит с настоящего устройства. Предоставляется гарантия – 1 год. Иногда устраиваются акции, которые помогут существенно сэкономить на приобретении.
Сайт https://resreg.ru представляет собой уникальную и высокотехнологичную платформу, объединяющую рестораны и заведения общепита, для которых качество остается в приоритете. Сервис предоставляет ценные и уникальные инструменты, которые предназначены специально для повышения качества обслуживания, а также эффективного и рационального управления своим заведением. Принимайте участие в реферальной программе и получайте бонусы. С этим сайтом у вас получится быстро и легко найти рестораны, посмотреть меню, рейтинги.
sildenafil 20 mg tablet
купить диплом ссср https://www.man-diploms.com .
Климат Бэст осуществляет продажу и установку проверенной техники. Предоставляются скидки постоянным клиентам, часто проводятся акции и распродажи. Для заказа товара не нужно выходить из своего дома, достаточно оформить заявку по телефону. Ищите кондиционер подольск? Climat.best – сайт, где можете в любое время ознакомиться с описанием каждой модели кондиционера. Грамотный консультант с удовольствием поможет подобрать лучший климатический вариант. Ощутите преимущества и удобство совершения выгодных приобретений в онлайн-магазине!
https://kursovyesociologiya.ru
generic viagra cost
https://referatymehanikagruntov.ru
Discover the Thrill of Jeetbuzz Casino: Play, Win Today
jeetbuzz casino jeetbuzzcasino.net .
Homemade mature porn 84 225 free sex videos pornsos
http://perfect.hanging-breasts-arab.bismillah.alypics.com/?regan-miya
self pic porn gallery lily from hannah montana porn pics exgirlfriends porn modern porn movies 3d father and son porn
https://referatymikroekonomika.ru
Разборка и снос старых деревянных и кирпичных домов с вывозом мусора в Москве и Подмосковье. Демонтаж деревянного дома – больше об услуге на сайте kleinburd.ru. Производим демонтаж домов, зданий и фундаментов вручную и спецтехникой по ценам ниже рынка за 24 часа. Бесплатный выезд инженера на объект.
https://kontrolnyepolitologiya.ru
На сайте https://ru-tile.ru приобретите сайдинг, а также фасадные панели, водосточные системы и различные сопутствующие товары, которые обязательно вам пригодятся. Вся продукция представлена только популярными, надежными брендами, которые отвечают за качество и дают гарантии, предоставляют сертификаты на продукцию. И главное, что пользоваться ей абсолютно безопасно. Посетите блог для того, чтобы ознакомиться с любопытными тематическими статьями. Фасадные панели представлены в самой разной цветовой гамме, что позволит подобрать решение на свой вкус.
sildenafil canada
Ваш надежный помощник в организации похорон
сервис организации похорон http://www.pohoronnoe-bjuro-444.ru/ .
amazon viagra
Выкуп автомобилей https://vykupautomobiley.ru/ стал еще выгоднее! Наша компания предлагает услуги по срочному выкупу любых автомобилей. Мы выкупаем автомобили в любом состоянии и с любыми проблемами. Наши специалисты проведут оценку вашего автомобиля и предложат лучшую цену на рынке. Обращаясь к нам, вы сэкономите свое время и силы, а также получаете профессиональный подход и юридическое сопровождение.
HDRezka https://hdrezka.ee/ это смотреть фильмы, сериалы, мультфильмы онлайн в хорошем качестве или скачать бесплатно. Все жанры, новинки 2023 и 2024 года на сайте. Вы также можете смотреть в отличном качестве на мобильном устройстве без скачивания. Ежедневное пополнение фильмов, сериалов, мультиков.
https://kupavnacity.ru/ сайт женский журнал
Ищете работу по запросу «подработка для молодежи» в Питере? У нас вы отыщите самые актуальные вакансии в СПб, где размещают работодатели РФ проверенные. Часто требуются промоутеры на раздачу листовок на улице, мерчендайзеры, модели. Ищите, где требуется работа для молодежи СПб? VK.com/youjob – здесь можете оставить свое резюме, если хотите найти работу. Вступите в сообщество, где подписано большое количество людей. Активность довольно высокая, поскольку вакансии размещаются ежедневно. Тут вы найдете то, что ищите, откликайтесь на предложения и приглашение к сотрудничеству вы получите быстро.
Мастер-классы по кондитерским украшениям: искусство, доступное каждому
пекарь кондитер обучение https://kursy-konditera-moskva.ru .
На сайте https://t.me/s/twin_russia вы сможете получить всю самую актуальную, точную и новую информацию, касающуюся БК Twin. Теперь вся подробная информация находится в вашем мобильном телефоне. В этом казино вас ожидает огромное количество побед, положительные эмоции, радость от игры! Если вы будете играть постоянно, то сможете рассчитывать на получение крупного приза. Это ваша возможность справиться с финансовыми трудностями. А еще регулярно выкладываются промокоды для вашей экономии. Не забывайте заходить на портал, чтобы получить свежие новости.
На сайте https://fankino.ru/ ознакомьтесь с новостями из мира кино и сериалов. Имеются интересные статьи о сюжете, содержании всех серий, чем закончится, актерах и ролях и многом другом, что вызовет интерес. Обязательно ознакомьтесь с викторинами, публикациями, посвященными праздникам и народным приметам. Также присутствует раздел повещенный ответам на вопросы по школьным конкурсным заданиям. Регулярно публикуется информация ко дню города и программе мероприятий на празднование дня города. Весь материал актуальный, интересный и качественный, а потому читать его приятно. Если и вы любите фильмы и сериалы, то прочтите о них прямо сейчас.
Viagra Soft Tabs 50mg
when viagra generic
https://kontrolnyepravovedenie.ru
https://resheniezadachpolitologiya.ru
amazon viagra
кракен даркнет – blacksprut даркнет, Кракен ссылка
Хотите сделать в квартире ремонт? Тогда советуем вам посетить сайт https://stroyka-gid.ru
где вы найдете всю необходимую информацию по строительству и ремонту. Если вам интересна тема – Строительство и ремонт дома своими руками, то вам просто необходимо посетить на котором, Вы прочтете много познавательных статей о строительных материалах, ремонте, перепланировке, покраске, клейке, укладке и конечно же утеплении.
https://resheniezadachpravovedenie.ru
Brand Viagra 25mg
Выкуп автомобилей https://vykup-automobiley-msk.ru/ – это быстро и выгодно! Если вы хотите продать свой автомобиль, но не хотите тратить много времени и сил на поиски покупателя, то обращайтесь к нам! Мы предлагаем услуги по выкупу автомобилей в любом состоянии: битые, кредитные, с проблемами по документам и т.д. Наши специалисты быстро и качественно проведут оценку вашего автомобиля, а также помогут с оформлением всех необходимых документов. Мы предлагаем самые выгодные цены на выкуп автомобилей, так что вы не пожалеете о своем выборе!
Вам интересны аккаунты Huawei Developer? ZennoLab предоставляет для вас аккаунты высшего качества, их реализация выполняется по заманчивым ценам. Аккаунты доступны как оптом, так и в розницу, купите их уже сейчас. Имеются аккаунты Google Console, Apple Developer, Google Voice, а также Huawei Developer. Ищите где купить huawei developer? ZennoLab.com – сайт, где размещена контактная информация, связаться с проверенным продавцом можно прямо сейчас, он предоставит больше информации и поможет с оформлением заказа. Приобретайте по выгодной цене аккаунты Huawei Developer на ZennoLab уже сегодня!
sildenafil pills
Агентство недвижимости Манхэттен https://manhatten2017.ru/ — купля, продажа, обмен, аренда жилой и коммерческой недвижимости в Чехове.
Anwap https://anwap.io/ это кинотеатр, в котором можно смотреть фильмы, сериалы, мультфильмы, аниме онлайн в хорошем качестве без скачивания и регистрации. Подборки фильмов, ТОП просмотров, ТОП по рейтингам – это удобный способ смотреть все самое новое за 2024 год и фильмы прошлых периодов.
https://admsvetlogorie.ru/blog/kak-poluchit-grazhdanstvo-chehii-dlya-rossiyan
гражданство чехии
Информация о способах излечения болезней хребта да суставов. Лечение остеохондроза, грыж МПД,
https://spinet.ru радикулита, артрита а также остальных заболеваний
viagra no prescription
На сайте https://prodemio.ru/ представлена исчерпывающая информация, которая касается ремонта автомобилей. Также подробно рассматриваются поправки в Законе, а также нововведения для автовладельцев. Они помогут понять, что допустимо делать, а за что грозит серьезный штраф. Есть информация про то, как выбрать электросамокат, имеются и обзоры автомобилей различных марок. Есть и другие статьи, которые помогут провести авторемонт двигателя, МКПП, ГУР, электрооборудования и подскажут, как устранить поломку машины быстро и надежно. Статьи написаны экспертами, поэтому они точно помогут вам.
На сайте https://lovely-professional.ru/ имеется вся необходимая продукция, которая создана для оформления бровей, ресниц. Представлен клей, препараты, различные аксессуары, а также другая продукция, предназначенная для салонов красоты, частных мастеров, а также тех, кто специализируется именно на этой сфере. Регулярно в интернет-магазине появляются новинки от ведущих брендов и высокого качества. Они вызовут интерес у всех лэшмейкеров. Магазин любит радовать своих клиентов, а потому постоянно проводятся акции.
Sildenafil 200mg
Курсы по продвижению сайтов: ключ к высоким позициям сайта
seo оптимизатор обучение https://kursy–seo.ru .
generic name for viagra
Где найти лучший клининг в Москве: обзор рынка услуг
топ клининговых компаний москвы https://www.kliningovye-kompanii-reiting1.ru/ .
buy viagra in mexico
купить диплом в Москве https://orik-diploms.com .
Viagra Professional 50mg
hello-food.ru
sildenafil for women
Предлагаем услуги по вскрытию замков дверей https://vskrytie-zamkow-service.ru/ любой сложности. Обеспечиваем быстрый и безопасный доступ в помещение. Наши специалисты имеют большой опыт работы и высокую квалификацию, что гарантирует качественный результат. Приятные цены вас удивят, а срочный выезд не заставит долго ждать.
buying generic viagra online
Сайт Hyperione на странице https://hyperione.com/ станет незаменимым помощником в вопросах использования социальных сетей и ПК, ноутбука и телефона. Информационный портал содержит статьи, инструкции и полезные советы. Тексты, опубликованные на сайте, посвящены использованию ПК, браузеров и социальных сетей, их особенностям. Дополнительно статьи сопровождаются понятными иллюстрациями, помогающими лучше разобраться в теме. Благодаря тематическому меню с разделами, содержащими тексты про определенные сайты и браузеры, намного удобнее ориентироваться в контенте.
Viagra 50mg
Sildenafil
viagra meaning
SEO обучение: курсы как увеличить продажи через поисковую оптимизацию
курсы seo и продвижение сайтов http://kursy-seo1.ru/ .
Зайдіть на сайт https://kompromis.info/ де ви зможете ознайомитися з найсвіжішими новинами економіки, політики, новинами спорту, авто новинами та багато іншого. Стрічка постійно оновлюється, що дозволить вам бути в курсі свіжих подій щодня. Також ви можете дивитися фото та відеоматеріали.
cheap price viagra
Не знаете, где найти аккаунты Huawei Developer? Вы точно попали по адресу. ZerocoolPro.Biz предлагает вам приобрести по недорогой цене аккаунты высочайшего качества. Они помогут вам расширить собственный бизнес и достичь успеха. Можете уже сейчас связаться с проверенным продавцом для получения дополнительных данных. Ищите где купить аккаунт huawei developer? Zerocoolpro.biz – сайт, где есть возможность узнать контактную информацию, здесь вам с удовольствием помогут в оформлении заказа. Покупать аккаунты Huawei Developer на ZerocoolPro.Biz – выгодно, убедитесь в этом лично. Совершается сделка прозрачно и безопасно.
https://kursovyelogistika.ru/
Brand Viagra 25mg
Услуга по вскрытию замков дверей в квартире https://vskrytie-zamkow-rf.ru/ предоставляется профессионалами нашей компании. Мы используем только современное оборудование и инструменты, а наши специалисты имеют высокую квалификацию и большой опыт работы. Мы гарантируем быструю и качественную работу, а также срочный выезд для наших клиентов.
Демонтаж, разборка и снос старых частных домов с вывозом мусора в Москве и Подмосковье под ключ. Разобрать дом в Московской области – здесь подробнее об услуге: 1ecenter.ru. Демонтируем дома, здания, строения и фундаменты вручную и спецтехникой по ценам ниже рынка за 24 часа. Бесплатный выезд инженера на объект.
order viagra online
https://referatymehanikagruntov.ru/
generic of viagra
Visit the website https://shopogolic.net/en which will help you make international purchases with delivery to your address. The best rates for parcel delivery from the UK, USA, Israel and Europe. We ship worldwide and help you buy on eBay, Amazon and other sites. Find out delivery rates on the website.
Краудлендинговые платформы: как выбрать лучшую для инвестирования?
краудлендинг чем отличается от кредита http://kraudlending77.ru/ .
Viagra Professional 50mg
otc sildenafil
where can i buy viagra without a prescription
Услуга по срочному вскрытию замков https://vskrytie-zamkov-rf.ru/ может потребоваться в различных ситуациях: потеря ключа, сломанный замок, заклинивший замок или попытка взлома. Наша компания предлагает услуги профессионального вскрытия замков любой сложности без повреждения двери. Только современное оборудование, гарантия качественна работ, результат. Мы готовы выехать на вызов в любое удобное для вас время, обеспечивая быстрое и безопасное вскрытие замка.
Выкуп автомобилей https://vykupavto.xyz/ удобная и быстрая услуга для автовладельцев, желающих быстро продать транспортное средство. Наша компания, занимающаяся автомобильным выкупом, предлагают объективную оценку стоимости автомобиля, оформление всех необходимых документов и мгновенную оплату. Это оптимальный вариант для тех, кто ценит свое время и желает совершить сделку без лишних хлопот.
купить диплом техникума http://www.landik-diploms.com .
Sildenafil
Мой опыт обращения к магу с приворотом в Германии – ясновидящая в германии отзывы
Живу я в небольшом городе в Германии, и найти среди кучи «магов» хорошего, трудно особенно в Германии. Намучилась я с этим очень. И как бывает, все решается на пьяную голову. Сидели с двоюродной сестрой, поведала ей свою проблему с мужем и о бесконечных поисках магов. Она очень удивилась, что я сразу не стала искать человека в России с Якутии или другого региона Дальнего востока – это можно сказать родина многих сильных шаманов и колдунов.
Порекомендовала мага, с которым сама работала. Поработала с ним и я.
Поэтому хотела бы написать отзыв о привороте на мужа, проведенном магом Романом Петровичем.
___________________________________________________________________________
Я хочу поделиться своим опытом использования услуг мага Романа Петровича с сайта https://cmag666.ru Ватсап 8 (984) 286-12-65
___________________________________________________________________________
Перед тем, как обратиться к нему, моя ситуация с мужем казалась мне безвыходной. Наш брак испытывал серьезные трудности, и наши отношения становились все более напряженными.
Мой муж, с которым мы прожили много лет, казался мне все более отстраненным и равнодушным. Он уходил в себя, избегал общения, а наши разговоры становились все реже и поверхностнее. Я испытывала огромную боль от того, что наша семья распадается, и что я теряю своего мужа. В итоге он еще и любовницу завел.
После долгих раздумий и поиска решения проблемы, я решила обратиться к магу Роману Петровичу за помощью.
Он провел для меня приворот на мужа – на все ушло 5 дней, и еще дал ряд простых рекомендаций, которым нужно было следовать до получения результата. Последовали дни ожидания
На 5 день после проведения приворота я почувствовала изменения в поведении мужа. Он стал проявлять больше внимания и заботы, мы снова начали общаться и находить общие интересы. Стали возвращаться чувства и заинтересованность мной как женщиной. Все быстро нормлизовалось в наших отношениях, с любовницей он порвал все контакты.
Сейчас, спустя несколько месяцев после проведения приворота, я вижу, что наш брак стал крепким, стабильным и счастливым, как и раньше! Мы с мужем снова чувствуем себя близкими и любящими людьми.
Я благодарна магу Роману Петровичу за его помощь и поддержку в трудный момент!
Теги gadalka v germanii отзывы – маг в германии отзывы
ясновидящие в германии отзывы – приворот в германии отзывы
маг в германии отзывы – маг в германии отзывы
Зайдите на сайт https://cryptograb.io/ и познакомьтесь с CryptoGrab CPA Affiliate Network, которая предлагает лучшие предложения в сфере криптовалюты с полным контролем и открытостью, для получения максимальной прибыли с криптотрафика. Мы предоставляем автоматический инструмент для обработки любых токенов и монет. Все наши инструменты полностью автоматизированы.
sildenafil buy
Pingback: lexapro alcohol interaction
cost of viagra at costco
Viagra 120mg
buying viagra online
На сайте https://splitkuban.com/ вы сможете купить сплит-системы и кондиционеры в Новороссийске по выгодной цене с доставкой и установкой. Зайдите в каталог, выберете тип сплит систем и узнайте цены на товары. Торгово-монтажная компания Южный холод также занимается обслуживанием, ремонтом и заправкой сплит-систем и кондиционеров.
Клиника инновационной хирургии предлагает большой спектр услуг. Ведут прием врачи различного профиля, они обладают высокой квалификацией, имеют достаточный опыт работы и положительные отзывы пациентов. Для проведения диагностики специалисты используют новейшее оборудование, вы можете быть уверены в качестве и эффективности лечения. Требуется записаться на прием к врачу гинекологу? Kix-Med.ru – клиника инновационной хирургии, где можно записаться на консультацию, укажите свое имя, контактный телефон и нажмите на соответствующую кнопку. Клиника позаботится о вас, обращайтесь!
Epic Wins Await: Discover the Magic of Glory Casino Now
glory casino apk glorycasinoapp.download .
Зайдите на официальный сайт Вадима Зеланда https://zelands.ru/ чтобы узнать больше о трансерфинге реальности и теории трансерфинга, который является уникальной технологией достижения целей и управления событиями, а сам Вадим Зеланд – считается автором номер один в российской эзотерике и самый читаемый современный писатель. Узнайте больше на сайте.
Сломаем, разбререм и демонтируем старые деревянные и кирпичные дома с вывозом строительного мусора в Москве и Московской области спецтехникой и вручную. Разборка дома – больше об услуге: artguns.ru. Сносим дома, здания и сооружения, постройки и фундаменты по цене ниже рыночной. Бесплатный выезд инженера на объект.
sildenafila
Hello from Happykiddi.
Проекты каркасных домов под ключ: какой стиль выбрать?
каркасные дома под ключ http://www.karkasnye-doma-pod-klyuch0.ru/ .
viagra prescription cost
https://skupka-stiralnyh-mashin.ru/
Viagra Professional 100mg
Выкуп авто у нас https://vykup-avto-moskow.ru/ это быстрый и удобный способ продажи транспортного средства. Мы специализируемся на оперативном выкупе авто, самостоятельно организуют оценку, диагностику и оформление всех необходимых документов. Вы получаете деньги сразу после заключения сделки. Полное юридическое сопровождение!
Зайдите на сайт https://easypayments.online/ который предлагает комплекс услуг для международных платежей для бизнеса и физических лиц. Easy Payments это комплексные решения для выхода бизнеса на зарубежный рынок: регистрация компаний, открытие счетов, платежные системы, аккаунты на маркетплейсах, консалтинг. Подробнее на сайте.
viagra meaning
cost of viagra at costco
купить диплом цена https://arusak-diploms.com .
strongest ed medication
buying ed drugs online
best ed drugs over counter
Мой опыт обращения к магу с приворотом в Германии – ясновидящая в германии отзывы
Живу я в небольшом городе в Германии, и найти среди кучи «магов» хорошего, трудно особенно в Германии. Намучилась я с этим очень. И как бывает, все решается на пьяную голову. Сидели с двоюродной сестрой, поведала ей свою проблему с мужем и о бесконечных поисках магов. Она очень удивилась, что я сразу не стала искать человека в России с Якутии или другого региона Дальнего востока – это можно сказать родина многих сильных шаманов и колдунов.
Порекомендовала мага, с которым сама работала. Поработала с ним и я.
Поэтому хотела бы написать отзыв о привороте на мужа, проведенном магом Романом Петровичем.
___________________________________________________________________________
Я хочу поделиться своим опытом использования услуг мага Романа Петровича с сайта https://cmag666.ru Ватсап 8 (984) 286-12-65
___________________________________________________________________________
Перед тем, как обратиться к нему, моя ситуация с мужем казалась мне безвыходной. Наш брак испытывал серьезные трудности, и наши отношения становились все более напряженными.
Мой муж, с которым мы прожили много лет, казался мне все более отстраненным и равнодушным. Он уходил в себя, избегал общения, а наши разговоры становились все реже и поверхностнее. Я испытывала огромную боль от того, что наша семья распадается, и что я теряю своего мужа. В итоге он еще и любовницу завел.
После долгих раздумий и поиска решения проблемы, я решила обратиться к магу Роману Петровичу за помощью.
Он провел для меня приворот на мужа – на все ушло 5 дней, и еще дал ряд простых рекомендаций, которым нужно было следовать до получения результата. Последовали дни ожидания
На 5 день после проведения приворота я почувствовала изменения в поведении мужа. Он стал проявлять больше внимания и заботы, мы снова начали общаться и находить общие интересы. Стали возвращаться чувства и заинтересованность мной как женщиной. Все быстро нормлизовалось в наших отношениях, с любовницей он порвал все контакты.
Сейчас, спустя несколько месяцев после проведения приворота, я вижу, что наш брак стал крепким, стабильным и счастливым, как и раньше! Мы с мужем снова чувствуем себя близкими и любящими людьми.
Я благодарна магу Роману Петровичу (Ватсап 8(984)286-12-65)за его помощь и поддержку в трудный момент!
Теги приворот в германии отзывы – гадание в германии отзывы
ясновидящие в германии отзывы – ясновидящая в германии отзывы 8(984)286-12-65
экстрасенсы в германии отзывы – гадание в германии отзывы
Наша компания https://vykup-auto-ru.ru/ предлагает услуги срочного выкупа любых автомобилей в любом состоянии. Наши специалисты проводят быструю и качественную оценку стоимости автомобиля, после чего вы сразу же получаете всю сумму наличными. Мы гарантируем юридическую прозрачность сделки и оформление всех необходимых документов. Не теряйте время на поиски покупателей, обратитесь к нам и получите максимальную выгоду от продажи вашего автомобиля!
https://1-win-casino.kz/ веб-сайтына өтіңіз, онда сіз 1win slot ресми веб-сайтына шолу, 1win kz артықшылықтары, бонустары, 1win login қалай тіркелу керек және қолжетімді ойындардың тізімімен танысасыз. 1win casino kz, сондай-ақ ұтыстарды қалай салуға және алуға болады 1 win және кез келген құрылғыларға арналған мобильді нұсқаға шолу 1 win kz.
generic ed drugs india
Отзыв о привороте, и о маге сделавшем приворот.
+
+
Были проблемы с мужем довольно давно. А в последний год отношения стали сыпаться очень быстро, все разурушилось до конца буквально на глазах. Особенно с появлением любовницы, это была коллега сработы. А у нас двое детей. Их я и сама могла обеспечить более чем! Но вот любила мужа, и хоте, чтобы дети росли с рожным отцом.
Обращалась много к кому, но доверия не было даже при первом общение. Случайно встретились с давней подругой. Разговорились о проблемах. Она посоветовал сильного мага с Дальнего Востока.
Обратилась к нему за приворотом, хотя были конечно сомнения. Звать его Роман Петрович. Берет за работу не сильно много по сравнению с другими, делает все быстро и в срок, не продает и всегда отвечает на возникающие вопросы. Вернул мужа мне за 7 дней, работу проводил 2 дня.
Очень довольна!
________________________
Обратиться к нему можно через сайт https://cmag666.ru или ватсап 8 (984) 286-12-65 – это настоящий маг и просто добрый человек, отлично знающий свое дело!
____________________________________________________________________________________
Сайт ищут по тегам #
# Ясновидящая # маге # отзывы # экстрасенс # гадалка
приворот людмила
лара таро 777 дзен
жанна таро доля ютуб
магия россия
гадалка зеленоград нелли отзывы
ольга холмова ютуб
борисов отзывы
отзыв гадалка маг эзо чат
юсупова анна николаевна
кто такой самир али отзывы
сайт бабы нины отзывы
натта наталья викторовна отзывы
ведьма александра
мария добрый
фото ведьмочек из тик тока
мастер никифор в контакте
приворот проверенный маг
best ed drug
Агентство недвижимости “Бутово” https://butovo-realty.ru/ является региональным отделом более крупного московского агентства недвижимости “My City”.
Зайдите на сайт Юген https://yugenjp.ru/ где вы сможете заказать уникальные товары из Японии. Купим и доставим товары в любую точку мира – керамика, свитки, живопись, чай, одежда и аксессуары, ножи, доспехи, нэцке и многое другое. Мы можем выкупить и доставить товар как из указанного вами интернет магазина, так и осуществить поиск товаров под запрос. Подробнее на сайте.
https://perevod-statey.ru/
Generic Ed Drugs Fda Approved
cheap ed medications online
best ed treatment
Срочное вскрытие замков https://vskrytie-zamkow-msk.ru/ это наша услуга, которая может потребоваться в различных ситуациях. Например, если вы забыли ключи дома и не можете попасть в свою квартиру или автомобиль. Также эта услуга может понадобиться, если замок сломался или если дверь была заперта изнутри и никто не может открыть ее обычным способом. Важно отметить, что срочное вскрытие замков производится профессионалами, которые имеют необходимые навыки и оборудование для выполнения этой работы.
best male enhancement pills
Выгодный выкуп автомобилей: быстро, просто и без хлопот на сайте https://vykup-avto-msk.ru/ Ищите надежный и выгодный способ продать автомобиль? Наша компания предлагает услугу выкупа авто на выгодных условиях! Мы осуществляем срочный выкуп любых автомобилей в любом состоянии, независимо от года выпуска, модели и пробега. Деньги сразу: после оценки и согласования стоимости автомобиля, вы получаете всю сумму.
Зайдите на сайт https://squid-game-zetflix.online/ где вы сможете смотреть онлайн в высоком качестве сериал Игра в Кальмара все сезоны, а также узнать все самые свежие новости о сериале. Смотрите онлайн бесплатно, наслаждайтесь неожиданными поворотами нового сезона и будьте в курсе последних серий — все на русском языке, все в HD-качестве.
best ed drugs
накрутка подписчиков в инстаграме дешево и быстро https://yeslike.ru/
ed drugs without prescription
ЖК «Солнечная аллея» https://s-alley.ru/ – официальный сайт застройщика. Квартиры в новостройках комфорт-класса в Зеленограде. Выгодно купить квартиру от застройщика в строящемся.
https://1-win-online.com/ veb-saytiga tashrif buyuring, u erda siz 1win cazino haqida to’liq ma’lumotni o’rganishingiz mumkin. Qanday o’ynash kerak, 1 win bonusi nima, 1vin qanday ro’yxatdan o’tkazish, 1win mobil ilovasini qanday yuklab olish va boshqa ko’p narsalar veb-saytda mavjud.
best over the counter ed pills
ed drugs online
erectile dysfunction medications
https://megakupitkvartiru.ru/
generic ed drugs india
buying ed pills online
Компания МЦК https://gk-dsg.com/ – ваш надежный партнер в строительстве. Мы предлагаем широкий ассортимент качественных строительных материалов и профессиональных.
Крайне рекомендую сайт под ключ от https://hidehost.net/ – лучшие в этой сфере.
best medicine for erectile dysfunction without side effects
1win casino
Наша компания https://vskrytie-zamkow.ru/ предоставляет услуги по срочному вскрытию замков любой сложности. Мы имеем большой опыт работы в данной сфере и используем только профессиональное оборудование. Наши специалисты оперативно выезжают на место и вскрывают замок без повреждения двери. Мы гарантируем качество нашей работы и полную конфиденциальность. Обратившись к нам, вы можете быть уверены в быстром и безопасном вскрытии замка.
Срочный выкуп автомобилей https://srochnyjvykupavto.ru/ это быстро, просто и выгодно! Если вы хотите продать свой автомобиль, но не хотите тратить время на поиск покупателя, обращайтесь к нам. Мы предлагаем услуги по срочному выкупу автомобилей в любом состоянии. Наши специалисты оценят ваш автомобиль и предложат лучшую цену. Мы работаем с автомобилями всех марок и моделей. Обращаясь к нам, вы экономите свое время и деньги.
best treatment for erectile dysfunction
Получите ваш займ без отказа прямо сейчас: начните с заполнения заявки
займы под залог автомобиля без отказа zaym-na-karty-bez-otkaza.ru .
Хочу поделиться своим невероятным опытом работы с магом Романом Петровичем с сайта https://cmag666.ru. Когда я оказалась в сложной ситуации со своим любимым человеком, я чувствовала, что моя жизнь рушится. Он начал дистанцироваться, и я не понимала, что происходит. Но мне посоветовали обратиться к Роману Петровичу, и это был лучший шаг в моей жизни.
Роман Петрович выслушал мои проблемы и предложил мне кладбищенский приворот для восстановления наших отношений. Я полностью доверила ему свои чувства и приступила к ритуалу с надеждой на лучшее.
С каждым днем я замечала, как наша связь становится все крепче и глубже. Мы начали общаться более открыто и искренне, а мои чувства к нему стали только усиливаться. Благодаря кладбищенскому привороту, наша любовь стала еще более крепкой и стойкой.
Работа Романа Петровича действительно поразила меня. Его внимание к деталям и профессионализм впечатлили меня. Благодаря его помощи я снова чувствую, что моя жизнь наполнена смыслом и радостью. Он настоящий мастер своего дела, и я безмерно благодарна ему за все, что он сделал для меня.
Я рекомендую Романа Петровича всем, кто столкнулся с проблемами в своих отношениях. Его помощь – это настоящий подарок судьбы, который может изменить вашу жизнь к лучшему. Спасибо, Роман Петрович, за вашу помощь и волшебство!
-отзывы людей кто делал кладбищенский приворот
-отзывы об кладбищенском привороте
-отзывы кладбищенский приворот
-кладбищенский приворот отзывы кто заказывал
-кладбищенские привороты отзывы
Besuchen Sie https://www.easycigs.de/, wo Sie elektronische Einwegzigaretten in großer Auswahl online mit schneller Lieferung in ganz Deutschland kaufen können. Wir bieten nur hochwertige Produkte und Zutaten an, ohne Kompromisse bei der Qualität einzugehen. Informieren Sie sich über die Preise und Lieferbedingungen auf der Website.
Вот мой реальный отзыв о заказе приворота на мужа.
_____________________________________________________
Я долго искала хорошего мага в Москве, но, кажется, все мои попытки были напрасными. Разочарованная и безнадежная, я стала обращаться к друзьям и знакомым за советом. И тут одна из моих подруг порекомендовала мне обратиться за помощью к магу с Дальнего Востока – Роману Петровичу с сайта.
Сначала я была немного скептически настроена – ведь удаленное заклинание казалось мне чем-то из области фантастики. Но моя подруга уверяла меня, что Роман Петрович – настоящий профессионал в своем деле. И я решила попробовать.
Когда я связалась с Романом Петровичем через его сайт https://cmag666.ru и рассказала ему о своей ситуации, он проявил искреннее понимание и сострадание. Он объяснил мне, как работает приворот, и какие изменения я могу ожидать. Я заказала у него приворот на моего мужа и начала ждать результатов.
Через несколько недель я заметила, что мой муж стал относиться ко мне по-другому. Он стал более внимательным и заботливым, и наши отношения начали испытывать явное улучшение. Я была поражена результатом!
Сейчас, когда я смотрю на нашу семью, я благодарна Роману Петровичу за его помощь. Он действительно знает свое дело и умеет помогать людям в трудных ситуациях. Если у вас есть проблемы в отношениях и вы ищете решение, я настоятельно рекомендую обратиться к Роману Петровичу – он сделает все возможное, чтобы помочь вам.
– приворот в москве кто делал
– сильный приворот в москве
– найти приворот в москве
– найти гадалку для приворота в москве
– кто делает приворот в москве форум
Клиника лечения боли https://imedicus.ru/ предлагает услуги квалифицированных врачей, в том числе невролога и нейрохирурга онлайн. Специализированный центр, занимающийся лечением головных и лицевых болей, болей в позвоночнике, мышцах и суставах это возможность получить квалифицированную консультацию, не выходя из дома. Подробнее не сайте.
generic ed drugs fda approved
https://vstx.ru/
Мой отзыв о том, как я заказал приворот у мага
В августе этого года я столкнулся с самым непредсказуемым испытанием – узнал, что моя жена в течение двух лет вела роман на работе после двадцатилетнего брака и троих детей. Наша семья была на грани разрушения, и я честно говоря, не знал, что делать в этой ситуации. Оба мы были виноваты, и мне казалось, что нет выхода. Тогда я обратился к Роману Петровичу через его страничку https://cmag666.ru , и могу сказать, что это было одним из самых лучших решений в моей жизни.
После того, как Роман Петрович провел расклад карт, который оказался точным от начала и до конца, у меня появилась надежда. Он подобрал магическую работу, а затем выполнен был ритуал с защитным щитом и привязкой на огонь (подробности можно найти на его блоге). Сегодня, в декабре, я рад сообщить, что благодаря его помощи я вернул любимого человека и спас нашу семью. Наши отношения перешли на качественно новый уровень во всех аспектах.
Однако, я хочу подчеркнуть, что магия не сделает всё за вас. Вам также придется приложить значительные усилия и измениться, если вы хотите добиться успеха. Перед Романом Петровичем я преклоняю колени – без его помощи я бы не был так счастлив. Если у вас возникли подобные проблемы, я настоятельно рекомендую обратиться к Роману Петровичу.
Сайт ищут по тегам
– что будет с тем кто заказал приворот
– заказать приворот на парня
– приворот заказать у мага
– заказала приворот
– как заказать приворот по интернету
Отзывы о приворотах в Екатеринбурге
—————————————–
Хочу поделиться своим незабываемым опытом работы с магом Романом Петровичем с сайта https://cmag666.ru. Проживая в Екатеринбурге, я столкнулась с трудностями в отношениях со своим мужем. Попытки решить проблемы с мужем при помощи местных магов не привели к желаемому результату.
Поиски настоящего мага привели меня на сайт Романа Петровича, и я решила попробовать снова. Будучи из Дальнего Востока, родины шаманов, Роман Петрович вызвал мое доверие, и я обратилась к нему за помощью в восстановлении чувств моего мужа.
С Романом Петровичем мы обсудили мою ситуацию, и он предложил мне кладбищенский приворот для возврата чувств моего мужа. Я решила довериться его опыту и приступила к ритуалу с надеждой на лучшее.
С каждым днем я замечала, как наши отношения становились все более гармоничными и близкими. Муж начал проявлять больше внимания и заботы, а его чувства к мне стали стойчивыми и глубокими. Благодаря кладбищенскому привороту, наш брак стал еще крепче и устойчивее.
Работа Романа Петровича действительно поразила меня. Его профессионализм и внимание к моей ситуации впечатлили меня. Благодаря его помощи я снова чувствую себя счастливой и уверенной в нашем будущем вместе. Он настоящий мастер своего дела, и я безмерно благодарна ему за все, что он сделал для нашей семьи.
Я рекомендую Романа Петровича всем, кто столкнулся с проблемами в своих отношениях. Его помощь – это настоящее чудо, которое может изменить вашу жизнь к лучшему. Спасибо, Роман Петрович, за ваше волшебство и преданность своему делу!
– приворот екатеринбург кто делает
– приворот в екатеринбурге кто делал
– приворот в екатеринбурге отзывы
– кто делал приворот в екатеринбурге
– екатеринбург приворот
most effective ed pill
Купить недорого https://plitkaruza.ru/ керамическую плитку, керамогранит, мозаику, клинкерные ступени в Рузе с доставкой по Тучково, Дорохово, Можайску, Звенигороду.
how to get a prescription for ed
Наша компания https://vskrytie-zamkow-24.ru/ предоставляет услуги по срочному вскрытию замков в любых ситуациях. Мы используем профессиональное оборудование, чтобы быстро и аккуратно вскрыть замок, не повреждая дверь. Наши специалисты имеют большой опыт работы и готовы выехать на вызов в любое время. Мы гарантируем качественную работу, профессиональный подход и даем гарантию на все выполненные работы.
erectile dysfunction drugs
ed tablets in india
cheap ed drugs online
Снос старых частных домов, разбор и вывоз мусора в Москве и области.
Наша компания предлагает услугу Снос домов под ключ. Мы работаем в Москве и Московской области. Мы осуществляем демонтаж домов, зданий, фундаментов как вручную, так и с использованием спецтехники. Наши цены ниже рыночных, и мы гарантируем выполнение работ за 24 часа. Бесплатно выезжаем на объект для оценки и консультации. Звоните или оставляйте заявку на нашем сайте, чтобы получить более подробную информацию и рассчитать стоимость услуг.
Зайдите на сайт https://gutschmid.ru/ автосервиса Gutschmid в Москве и ознакомьтесь с услугами центра, такими как: кузовной ремонт Москва, ремонт кузова после ДТП Москва, покраска автомобиля Москва, ремонт кузова автомобиля Москва, кузовной ремонт автомобиля. Все услуги оказываются профессионалами на самом современном оборудовании.
My Door Store https://mydoorstore.ru/ – интернет-магазин входных дверей с доставкой по Москве, Санкт-Петербургу и всей России. Замер и дешевая установка входной двери после покупки.
Наша фирма https://vskrytie-zamkow-365.ru/ специализируется на оказании услуг по вскрытию замков дверей. Мы используем только современные инструменты и оборудование, что позволяет нам быстро и аккуратно вскрывать замки любой сложности. Наши специалисты обладают большим опытом работы и высокой квалификацией, что гарантирует качество предоставляемых услуг. Мы также гарантируем срочный выезд для своих клиентов и гарантию на работы.
https://avidigital-spb.ru/
best medication for ed
поисковое продвижение seo
best ed drugs for women
highest rated ed medication
Pingback: ketamine/baclofen/bupivacaine/cyclobenzaprine/diclofenac/gabapentin
generic ed drugs india
Наша компания https://vskrytie-zamkov-365.ru/ предлагает услуги по вскрытию замков дверей. Мы используем современное оборудование и инструменты, что позволяет быстро и аккуратно открывать любые замки. Наши специалисты имеют высокий уровень квалификации и большой опыт работы, что гарантирует качество предоставляемых услуг. Мы также гарантируем полную конфиденциальность и стараемся выполнять работы в кратчайшие сроки.
https://doorsvnn.ru/ 3-я дверь или откос в подарок. Доставка дверей осуществляется бесплатно, Подъем на этаж бесплатно, Пенсионерам скидка до 30%.
best male enhancement pills
svetlanamag ru После множества неудачных попыток найти свою идеальную половинку, я обратилась к магу Светлане за помощью. Ее ритуал приворота помог мне встретить человека, с которым я почувствовала невероятную связь. Сейчас мы счастливы вместе, и я благодарна Светлане за ее помощь в поиске истинной любви.
Почта svetlanamagg @ yandex ru
-самые настоящие маги
-кто настоящий маг в россии
-реальные и проверенные маги
-проверенные маги отзывы
low cost ed drugs
Boost productivity with Upload to Google Drive https://chrome.google.com/webstore/detail/upload-to-google-drive/cjdcbcklfcmgifjlipdkhdkcdfnldiim – save your photos, videos, files and other documents directly to Google Drive.
ed drugs online canada
На сайте https://ziongpt.ai/ вы сможете воспользоваться таким уникальным информационным продуктом, как нейросети, в режиме реального времени и абсолютно бесплатно. Этот агрегатор направит вас на самые популярные и надежные нейросети, которые порадуют своей отлаженной работой. Они смогут нарисовать картинку, сгенерировать текст или даже поговорить с вами. Попробовать все это вы сможете прямо сейчас или в любое другое время. Большинство программ работает без подключения VPN. При этом не потребуется и иностранная карта.
ed pills cheap
Услуги https://vskrytie-zamkov-service.ru/ по профессиональному вскрытию замков дверей. Мы профессионалы, работающие в самые сжатые сроки. Моментальный выезд, современные инструменты, быстрый и безопасный способ вскрытия, гарантия. Высокая квалификация гарантирует качественный результат.
best ed drugs over counter
Дача “Дом-Баня” https://arenda-dacha.ru/ расположена в Подмосковье. (80 км к югу по Симферопольскому шоссе), построена в 2010 году.
erection pills viagra online
Секреты выбора тарифа: безлимитный интернет плюс качественная мобильная связь
безлимитный интернет для смартфона https://mobilnyj-bezlimitnyj-internet.ru .
best non prescription ed treatment
Сайт https://fasonika.shop – ваш надежный партнер в мире стиля и элегантности. Мы специализируемся на продаже высококачественной женской верхней одежды, создавая коллекции, которые сочетают в себе последние тренды. Наша миссия – подчеркнуть вашу индивидуальность. Мы предлагаем широкий ассортимент верхней одежды: от элегантных пальто и дубленок до стильных курток и пуховиков. Наши дизайны вдохновлены последними тенденциями моды, при этом мы уделяем особое внимание качеству материалов и деталям.
На сайте https://suligarent.ru/ вы сможете заказать услуги по аренде авто. Аренда авто на сутки или почасовая аренда в Калининграде, со страховкой, для работы в такси или для корпоративных клиентов это удобный способ передвигаться, не имея собственного автомобиля. 14 лет опыта, круглосуточная тех поддержка, лучшие цены, большой парк авто и пробег без ограничений!
best ed drugs for women
Росстранс Экспедиция https://rosstrans.ru/ ведущая транспортная компания в Москве, специализирующаяся на оказании широкого спектра логистических услуг. Мы оказываем транспортные услуги по перевозке любых грузов автомобильным, морским, авиационным и железнодорожным транспортом. На сайте имеется калькулятор расчета доставки. Подробнее на сайте.
История-отзыв о привороте у мага Александры (magpa666 @ bk ru) – magpomosh ru
В моей жизни наступило трудное время, и я решил обратиться за помощью к магу Александре с сайта magpomosh.ru. Моя семейная жизнь находилась на грани разрыва, и я не знал, что делать. Ситуация казалась безвыходной, и я чувствовал себя очень одиноким.
С момента первой встречи с Александрой я понял, что попал по адресу. Она внимательно выслушала мои проблемы и предложила мне ряд решений, среди которых был и ритуал приворота. Я решил попробовать, не имея особо больших надежд.
Но результат меня поразил. Спустя некоторое время после проведения ритуала наши отношения с женой начали улучшаться. Мы стали чаще общаться, лучше понимать друг друга, а наша семья стала крепче. Я благодарен магу Александре за ее терпение, помощь и понимание. Спасибо вам, Александра, за ваше волшебство!
Настоящий маг – magpa666 @ bk ru – magpomosh ru
_________________________________________________________
Сайт ищут по тегам:
есть ли маги настоящие сильные настоящие маги настоящий маг помог
как найти настоящего мага а не шарлатана приворот у настоящего мага подскажите настоящего мага
настоящие маги и ясновидящие проверенный маг москва как определить шарлатана от настоящего мага
сильнейшие маги настоящие маги настоящие шарлатаны как стать настоящим белым магом
После множества неудачных попыток найти свою идеальную половинку, я обратился к магу Роману Петровичу за помощью. Его Вуду-приворот с сайта cmag666.ru помог мне привлечь внимание девушки моей мечты. Сейчас мы встречаемся, и я не могу поверить, что все это стало возможным благодаря ритуалу Романа Петровича.
Запросы сайта в поиске
___________________________________________________________
приворот вуду отзывы кто делал кто делал приворот вуду отзывы приворот по вуду кто делал отзывы
отзывы о привороте вуду на куклу приворот на вуду кто делал отзывы приворот вуду отзывы
non prescription erection pills
Про отделку https://smesimels.ru/ – Ремонт и отделка: материалы и оборудование. Лак и краски, Интерьер Клей и герметики, Стройсмеси, Оборудование, Грунтовка.
what can i buy that works like viagra
Прекрасное гэмблинг-портал! pokerdom впечатлил изобилием игр, в особенности альтернативами карточных игр. Принимал участие в матчах – интригующий практика. Поощрения для начинающих и пromptные выплаты добавили бонусов. Рекомендую для любителей азарта и покерных забав!
ed drugs from canada
pills for ed online
https://best-santehnika.store/
На сайте https://ramenbet-dk.fun/ вы сможете сыграть в любопытное и интересное игровое казино, а также автоматы, покер, рулетку. Вы сможете рассчитывать на приятные бонусы, воспользуйтесь шансом получить крупный приз и различные вознаграждения уже сегодня. У вас получится насладиться огромным количеством различных бонусов, игрой с живыми дилерами. Регистрация максимально простая, займет не более пары минут, что значительно упрощает процесс. Она гарантирует доступ ко всем функциям для большего выигрыша.
Generic Ed Drugs Cost
ed pills for sale
Зайдите на сайт https://torgovoe-oborudovanie-rf.ru/ где вы сможете купить торговое оборудование на заказ. Изготовление торговой мебели в СПб только из качественных материалов с гарантией. Торговая мебель на заказ — это возможность получить как типовые проекты, так и индивидуальный проект. Торговая мебель в Санкт-Петербурге по приемлемым ценам от производителя, без посредников это возможность существенно сэкономить на комплексном оснащении магазина.
https://free-promocode.ru/
На сайте https://playpokerokrussia.ru/ вы сможете начать играть в покер онлайн. Скачай быстрый и технологичный клиент ПокерОК и играй с реальными людьми 24/7 и получай быстрые выплаты. PokerOK это безопасная игровая платформа, где в том числе проводятся постоянные турниры, а возможность играть с любого устройства это удобный способ играть в любом месте.
best over the counter ed pills
best medication for ed
impotence pills over the counter
Отзывы – Помогли сделать – приворот на женатого сильный https://cmag666.ru Ватсап 8 (984) 286-12-65
маг настоящего времени – настоящие маги есть ли они
ed meds
vavada казино онлайн произвела приятное воздействие! Интенсивный оформление, полнота азартных развлечений и заманчивые бонусы. Регулярные мероприятия привлекают, а успехи приятно изумляют. Обслуживание на высоком качестве. Рекомендую всем развлекательным игрокам!
Ed Drugs
erectile dysfunction pills from india
аэропорт хургада
erectile dysfunction medicine india
https://seo116.ru/
носимый видеорегистратор https://nagrudnievieoregistratori.ru/
На сайте https://shemi-otopleniya.ru/ ознакомьтесь с гибкой подводкой, которая выполнена из нержавеющей стали. При этом она может быть самых разных размеров и диаметра, чтобы отвечать требованиям. Изучите технические характеристики прямо сейчас. На сайте представлена информация и относительно того, где может применяться такая продукция, ее предназначение. Есть фотографии для того, чтобы наглядно продемонстрировать то, как устроена гибкая подводка. Она выполнена из современных материалов, которые не покрываются ржавчиной.
На сайте https://paketix.ru/ вы сможете легко заказать изготовление и печать бумажных, полиэтиленовых пакетов с логотипом на заказ. Типография Пакетикс производит пакеты с логотипом из бумаги, картона и других материалов, а большой ассортимент позволит каждому заказчику найти то, что ему необходимо. Подробнее на сайте.
best ed drugs for women
Услуга сноса старых частных домов и вывоза мусора в Москве и Подмосковье под ключ от нашей компании. Работаем в указанном регионе, предлагаем услугу разобрать дом цена. Наши тарифы ниже рыночных, а выполнение работ гарантируем в течение 24 часов. Бесплатно выезжаем для оценки и консультаций на объект. Звоните нам или оставляйте заявку на сайте для получения подробной информации и расчета стоимости услуг.
best non prescription ed medication
svetlanamag ru – svetlanamagg @ yandex ru
После развода с моим бывшим мужем я чувствовала себя потерянной. Но благодаря ритуалу приворота у мага Светланы, я встретила нового мужчину, который полностью меня понимает и ценит. Я счастлива вновь!
-сайты настоящих магов
-кто настоящий маг
-союз магов россии настоящие
-а есть ли настоящие маги
best male enhancement pills
non prescription ed pills
ed treatments that work immediately
очистка крыши от мха в Москве https://eco-life-group.ru/ochistka-fasadov/unichtozhit-mokh-na-krovle/
best ed pills that work
best treatment for ed in india
Зайдите на страницу https://printouch.ru/pakety-polietilenovye где вы сможете за минуту заказать изготовление полиэтиленовых пакетов. Поможем в подготовке макета, различная плотность, приятные цены, доставка – это удобный способ получить индивидуальные решения быстро и с отличным качеством. Подробнее на странице.
best online ed prescriptions
erectile dysfunction medicine india
На сайте https://xn—–6kcbbhfu0bfdcki4aej3b7a.xn--p1ai/ запишитесь на такую нужную услугу, как оклейка кузова. На выбор представлены самые разные варианты пленок, например, глянцевая, антихром, матовая, защитная, а также антигравийная. Перед вами огромный выбор вариантов, чтобы вы подобрали именно то, что необходимо. Опыт работы в данном направлении – более 10 лет. На все работы предоставляются гарантии. Установлены привлекательные расценки, чтобы работами воспользовались все желающие.
impotence pills over the counter
online ed drugs
generic ed drugs canada
ed drugs compared
Выкуп квартиры в кратчайшие сроки. Быстрая оценка стоимости и помощь в подготовке документов. Помощь с переездом.
выкуп квартир в санкт-петербурге
best ed pills non prescription
Жіночий блог MeatPortal розповість корисну інформацію з порадами і рекомендаціями ексертів для сучасних домогосподинь. На сайті https://meatportal.com.ua/ знайдете корисні публікації на теми: кулінарія, дім та сад, сім’я, здоро’я та інші.
best non prescription ed treatment
Астарт Вард отзывы magastartvard . ru Астарт Вард отзывы
______________________________________________________
Отзыв magastartvard . ru как он мне помог
Маг Астарт Вард помог мне снять негативные блокировки и привлечь любовь в мою жизнь. Его энергетическая работа была просто потрясающей! Я уже чувствую изменения в своих отношениях и благодарна за его поддержку.
______________________________________________________________
Астарт Вард отзывы форум
ed drugs in india
best over the counter ed medication
treatments for ed
Крастко – Отзывы magpomosh ru – сайт – чертогрыз отзывы о привороте
Отзывы сайт magpomosh .ru – маг Александра
Обратился к магу Александре за помощью в защите от негативных энергий. Ее защитные магические практики действительно помогли мне чувствовать себя более защищенным и уверенным.
_________________________________________________________
Сайт ищут по тегам:
маги россии проверенные настоящие маги найти настоящие маги и шарлатаны
адрес проверенного мага помог настоящий маг только настоящие маги
черные маги оплата по результату проверенные в москве сайт проверенных магов нет магов настоящих
что может настоящий маг и за какое время кому помог настоящий маг настоящий маг колдун
На сайте http://medtorg.top/ каждое медицинское учреждение получает возможность приобрести специальное оборудование, а также качественные расходные материалы, которые идеально подходят для использования в стационаре и дома. Вся продукция представлена лучшими, проверенными марками, которые работают специально для вас. В разделе вы найдете всю необходимую продукцию, предназначенную для искусственной вентиляции легких и многое другое. Очень часто в компании проходят акции, чтобы вы сэкономили бюджет.
ed pills non prescription
strongest ed medication
https://hidehost.net/
new erectile dysfunction treatment 2024
Зайдите на сайт https://allestate.pro/ где вы найдете актуальные объявления о продаже и аренде недвижимости в России и мире, а также новости недвижимости сегодня. Сайт — это полноценная платформа, где собраны все самые свежие объявления где можно купить, снять, обменять, оценить объект, а также почитать новости рынка недвижимости, последние новости, новости России.
раскрутка сайта seo
Чистота, которую вы заслуживаете: профессиональное мытье окон
сколько стоит помыть окна http://mytie-okon1.ru/ .
https://hidehost.net/
купить французское вино
Вам нравятся азартные игры с темой Древнего Египта? Значит слот Rise of RA от компании EGT точно для вас, он предоставляет промо коды и разные бонусы. Вы получите истинное удовольствие от игры. a href=https://rise-of-ra.com/>https://rise-of-ra.com/ – сайт, где вы узнаете, как сделать ставку в слоте, какой максимальный выигрыш в игре, как разыгрываются джекпоты и др. Посмотрите информацию прямо сейчас. Слот имеет удобное управление, вам понравится его дизайн и музыка. Он дает множество эмоций и приличные деньги. Рискуйте и играйте!
balloon казино – подлинный напиток волнения! Я окунулся в бурю интересных игровых автоматов и взорванных призов. Различие азартных развлечений завораживает, а вознаграждения удовлетворяют. Рекомендую всем желающим, кому-либо рвет эмоциональное игровую площадку!
Зайдите на сайт https://na-telefon.biz/ где вы сможете заказать голосовые поздравления с днем рождения по имени или отправить аудио поздравление по именам с днем рождения, а также всегда прикольные поздравления с днем рождения на телефон на нашем сайте. Зайдите, узнайте больше – получатель всегда будет рад необычному поздравлению.
На сайте https://avanta-avto-credit.ru/ вы сможете ознакомиться со всеми автомобилями, которые получится приобрести прямо сейчас. Воспользуйтесь выгодными предложениями от надежного и проверенного дилера «Аванта». Он готов предложить лучший автомобиль, который прослужит долгое время, обрадует своим идеальным техническим состоянием, а также маневренностью. Есть и новинки автомобилей, которые вы сможете приобрести с огромной выгодой. Для всех клиентов доступен быстрый подбор автомобиля по заданным параметрам.
Зайдите на сайт https://alfases.ru/ и вы сможете заказать уничтожение насекомых и грызунов в Уфе и услуги дезинсекции, дезинфекции и дератизации помещений. Служба уничтожения насекомых и грызунов в Уфе предоставляет большой комплекс различных услуг, с ценами на которыми вы сможете ознакомиться на сайте.
top ed pills https://fintryides.com/
раскрутка сайта сколько стоит
Что такое реконструкция и зачем она необходима? Почему важно различать капремонт и реконструкцию? Чтобы это все понять, необходимо прочитать нашу статью на сайте. Требуется реставрация дома? VC.ru – здесь есть возможность узнать, чем капремонт отличается от реконструкции объекта. «Артель и С» – компания, занимающаяся непосредственно реконструкцией, и готовая взяться за проект разной сложности. Доверяйте истинным профессионалам своего дела!
cheap ed pills from india https://fintryides.com/
Анапа Транспорт – компания, осуществляющая безопасные пассажирские перевозки. Здесь имеются самые разные автомобили, которые соответствуют высочайшим требованиям, они будут поданы в точное время. Транспорт комфортабельный, чистый и исправный, им вы останетесь довольны. Ищете заказать автобус в aнапе? Anapa-Transport.ru – сайт, где можете прямо сейчас посмотреть благодарственные письма. Водители все вменяемые и доброжелательные, они постоянно на курсах повышают квалификацию. Если вы нуждаетесь в качественном сервисе, смело обращайтесь в компанию «Анапа Транспорт».
На сайте https://belpapa.ru/ вы сможете изучить огромный ассортимент разнообразного сайдинга. Здесь вы найдете акриловый, виниловый, вспененный, формованный сайдинг, а также огромное количество комплектующих эталонного качества. Вы сможете подобрать решение под свои предпочтения, пожелания и абсолютно любого цвета, чтобы оно хорошо гармонировало. Для подбора оптимального решения необходимо воспользоваться специальным фильтром по определенным параметрам. Также воспользуйтесь фильтром по месту установки, имитации материала, производителю, цветовой гамме, размеру.
best ed drugs https://fintryides.com/
купить аттестат https://www.rudik-attestats.com .
non prescription male enhancement pills https://fintryides.com/
1хбет регистрация официальный
Хотите узнать секреты действенного продвижения от настоящих профессионалов? Добро пожаловать на наш ресурс, здесь рассматриваются способы, которые вы должны применять, пытаясь продвинуть сайт в гугле. Ищете seo google? VC.ru – тут представлены полезные рекомендации по оптимизации. У вас появились какие-то вопросы? Свяжитесь с нашими опытными специалистами, они вам помогут и предложат коммерческое предложение.
best treatment for ed in india https://fintryides.com/
best over the counter ed pills https://fintryides.com/
https://hidehost.net/
https://arm-wine.ru/
1xbet скачать приложение
Новости культуры и шоу
online ed doctor prescription https://fintryides.com/
Sushiap избавит вас от долгих часов у плиты и магазинов. Закажите доставку вкуснейшей пиццы, напитков, сочных бургеров, картофеля фри и другое. Здесь внимательно относятся к выбору продуктов, а также соблюдению их сроку годности. Доставка осуществляется точно по времени, все курьеры вежливые. Вы 100% останетесь довольны ценой, вкусом еды и сервисом. Ищете доставка еды на дом пятиморск? Sushiap.ru – сайт, где вы можете легко оформить заказ. Также у вас есть возможность позвонить по телефону. Положите в корзину то, что вам нравится, и не забудьте про десерт!
Известная транспортная компания «Открытый Краснодар» выполняет пассажирские перевозки по высшему качеству. Она предлагает клиентам оптимальные условия и очень ими дорожит. Доступная цена, своевременная подача, чистый салон, исправность транспорта – все это гарантируется. Ищете аренда минивэнов на 8 мест? Krasnodar-Transport.ru – сайт, где есть возможность по вашему маршруту получить индивидуальную консультацию. Также здесь указан номер круглосуточной диспетчерской службы. Водители доброжелательные, опытные и культурные, вы точно можете в них быть уверенными.
Зайдите на сайт https://eurasiaoptom.ru/ где вы сможете заказать оптом свежие фрукты и овощи из Египта. Мы организуем оптовые поставки от лучших ферм Египта. Весь ассортимент имеет сертификаты, а оплату налогов и сборов мы берем на себя. Ассортимент постоянно пополняется. Подробнее на сайте.
промокод 1xbet
игры на деньги – подлинный герой среди виртуальных игровых заведений! Увлекательные слоты, настольные развлечения и дружелюбная клиентская служба. После победы быстро получил свой награду. Это казино определенно знает, как делать счастливыми участников. Рекомендую каждому!
На сайте https://wowbanner.ru/ закажите расчет проекта в типографии «WOWbanner». Здесь оказываются услуги на высоком уровне, а все работы выполняются быстро, качественно, в минимальные сроки. Вам доступна печать любых форматов, в том числе, быстрая. А определить цену услуги можно и сейчас, воспользовавшись специальным калькулятором. Для того чтобы определиться с услугами, необходимо ознакомиться со всеми из них. Наибольшей популярностью пользуется широкоформатная, интерьерная печать, а также УФ, различная полиграфическая продукция.
Компания «BSH-Systems» собрала профессиональную команду, которая реализует проекты любой сложности. Квалифицированные и добросовестные специалисты понимают оборудование до малейших деталей. https://bestsmarthouse.ru/ – сайт, где есть возможность узнать, как сделать из обычного дома либо квартиры многофункционального помощника, при этом не потратив свои деньги зря. Ознакомиться с информацией можете в удобное для вас время. Компетентные менеджеры готовы ответить на вопросы и подобрать лучшую систему Умного дома. Обращайтесь в любое время!
На сайте https://beperfect-shop.ru приобретите профессиональные материалы для наращивания, а также ламинирования ресниц. В каталоге вы найдете огромное количество позиций, которые будут незаменимы для лэшмейкеров. Вся продукция производится именитыми, надежными брендами, которые учитывают предпочтения, профессиональные аспекты. В разделе вы найдете ресницы, клей для наращивания, а также дополнительные аксессуары. Установлены привлекательные и доступные цены. Очень часто проходят акции, которые позволят сэкономить. Специально для вас бесплатная доставка.
ed pills india https://fintryides.com/
Ищете актуальные новости игр различных платформ ПК, PS, XBox, Switch? Заходите на Pro100Gamers, тут у вас есть возможность узнать, как подключиться и настроить выделенный сервис Palworld, где найти в Enshrouded болгарский перец и другое. Авторы разрабатывают тщательно материал, обеспечивая вас нужной информацией. Ищете онлайн гайд по играм на пк? Pro100gamers.ru – популярный сайт, с помощью которого вы увеличите свои геймерские горизонты. Здесь предлагают обзоры новинок и публикуют последние игровые анонсы. Вы достигнете великолепных результатов в мире компьютерных развлечений!
Интернет магазин сантехники для ванной онлайн – Ванна Хоум https://vanna-home.ru/ это большой ассортимент сантехники и комплектующих российского и европейского производства с быстрой доставкой по Москве и области. Наш обширный каталог предлагает выгодные цены, скидки и бонусы. Узнайте подробнее на сайте.
Компания Такси и Трансфер в Анапе гарантирует прибытие автомобиля в указанный срок вне зависимости от каких-нибудь обстоятельств. Водитель может предоставить вам необходимую информацию – бесплатно. Есть возможность заказать машину с детским креслом. Ищете заказ машины такси? Anapa-Taxi-Transfer.ru – сайт, где есть возможность рассчитать стоимость поездки. Вся без исключения техника содержится в безупречном техническом состоянии. Здесь найдут транспорт, идеально подходящий для ваших нужд, не отказывайте себе в комфортном передвижении!
https://seo116.ru/
ed drugs without prescription https://fintryides.com/
Москва Оклейка Авто РФ предлагает высококачественные услуги. Здесь работают опытные мастера, которые любят свое дело. Результатом вы будете довольны, цена вас порадует. Если у вас возникли какие-либо вопросы, свяжитесь с нами по телефону, мы ответим на них. Ищете бронирование пленкой kia proceed? Москва-оклейка-авто.рф – сайт, где указаны актуальные цены на оклейку, ознакомиться с ними можно в любое удобное для вас время. Подписывайтесь на рассылку, чтобы сэкономить на покупке товаров либо воспользоваться выгодной скидкой на услуги.
Не знаете где найти межкомнатные двери? Интернет-магазин «Росдорс» то, что вам нужно. Приобретенные тут двери будут надежной защитой для вашего частного дома, квартиры либо другого помещения. Здесь клиентам предлагают приемлемые цены. Ищете продажа входных дверей? Rosdoors.ru – сайт, где представлен богатый ассортимент дверей в электронном каталоге с детальными описаниями и фотографиями каждой модели. Совершить покупку можно различными способами: связаться с компетентными специалистами по телефону либо использовать функционал «Корзины».
best over the counter ed medication https://fintryides.com/
Зайдите на сайт Компании WESTCOM https://westcom.ru/ которая предлагает большой ассортимент торгового оборудования и мебели для магазинов, офисов, гостиниц, торговых залов в наличии и на заказ. Большой ассортимент, нестандартные решения, акции и скидки – все это на нашем сайте. А удобный каталог товаров позволит ознакомиться с полным ассортиментом.
Желаете сделать вашу беседку еще более комфортной? Тогда вам стоит сделать заказ мягких окон у нас, они будут использованы удобно в любых погодных условиях. Мы готовы ответить на все вопросы и оказать помощь в подборе нужного решения для вашего объекта. https://pro-myagkie-okna.ru/ сайт, где вы можете с легкостью заказать расчет. Наши цены вас точно порадуют, ведь они доступные. Для своих клиентов мы обеспечиваем максимальное удовлетворение потребностей. Приложим все усилия для того, чтобы ваше пространство было истинным уголком комфорта, обращайтесь!
where to buy ed drugs online https://fintryides.com/
купить аттестат за 11 класс http://www.gruppa-attestats.com/ .
https://beckom.ru/
На сайте http://diplom-sale.ru/ вы сможете оформить дипломы на заказ и за небольшую плату. В компании работают первоклассные специалисты, которые дадут профессиональную консультацию и оформят все так, как нужно. Все документы государственного образца. При необходимости курьер привезет диплом туда, куда скажете. Также доступно и экспресс-оформление. Воспользуйтесь услугой прямо сейчас, чтобы получить профессию мечты. Компания давно находится на рынке, а потому отлично знакома со всеми потребностями клиентов.
На сайте https://spb.zabirator.ru/ закажите обратный звонок для того, чтобы воспользоваться такой важной услугой, как вывоз мусора на бесплатной основе. При этом работа проводится профессиональными грузчиками. Однако есть часть услуг, за которые придется заплатить, хоть и небольшую сумму. В этой компании получится вывезти бытовой, строительный мусор, металлолом, батареи отопления и многое другое. Автомобиль предоставляется абсолютно бесплатно. Демонтаж проводится на профессиональной основе, работают обученные грузчики.
МСТРОП предлагает новейшие грузоподъемные механизмы. В наличии текстильные, цепные, канатные стропы, стяжные ремни, скобы такелажные, домкраты, лебедки ручные, лента для строп и ремней, траверсы и др. Широкий ассортимент продукции позволяет удовлетворить запросы требовательных к качеству покупателей. Ищете симонова 11? M-Strop.by – сайт, где вы найдете каталог, посмотреть его можно уже сейчас. Оставить заявку на продукцию можно по контактному номеру телефона. Клиенты «МСТРОП» оставляют положительные отзывы о быстроте выполнения заказов.
medicine for ed in india https://fintryides.com/
https://seolinkedin.ru/
Интернет-магазин Bellawoman https://bellawoman.ru/ это итальянская брендовая одежда в Москве. Зайдите на сайт и ознакомьтесь с широким ассортиментом каталога, где каждая женщина и мужчина обязательно найдет для себя аксессуары на все случаи жизни, а акции на бренды позволят существенно сэкономить.
highest rated ed medication https://fintryides.com/
Наша компания https://tob.ru/ предлагает изготовление и продажу торгового оборудования и мебели для магазина, сетевых магазинов, торговых центров на заказ. Индивидуальные проекты и готовые решения. Ознакомьтесь с реализованными проектами на сайте или подберите готовые решения в каталоге. Собственное производство в Москве.
generic ed drugs over the counter https://fintryides.com/
Где взять микрозайм без переплат: секреты выбора
микрозайм деньги https://www.oformit-mikrozajm-onlajn.ru/ .
Предлагается продажа 2-х этажного коттеджа из сосны в Архангельской области на берегу реки Онеги. В нем имеется 9 комнат и все необходимые удобства. Ищете готовый коттедж купить? Усадьбыкаргополя.рф – сайт, где представлена окончательная стоимость всего коттеджа. Также здесь показан кадастровый номер участка. Отображен контактный номер телефона собственника, показана и электронная почта. Посмотреть фото и подробную информацию о коттедже вы можете уже сейчас.
Pingback: cephalexin for boils
Pingback: ciprofloxacin and penicillin allergy
ed dysfunction treatment https://fintryides.com/
Забудьте о низких позициях в поиске! Наше SEO продвижение https://seopoiskovye.ru/ под ключ выведет ваш сайт на вершины Google и Yandex. Анализ конкурентов, глубокая оптимизация, качественные ссылки — всё для вашего бизнеса. Получите поток целевых клиентов уже сегодня!
На сайте https://mesibot.agency/he/striptease закажите такую важную и популярную услугу, как организация праздников, включая масштабные свадьбы, дни рождения. Здесь вы сможете организовать незабываемый, яркий и интересный мальчишник либо девичник, пригласить стриптизерш. Такой праздник вы точно никогда не забудете, ведь он будет ярким, интересным и веселым. Важно то, что он обойдется вам по привлекательной стоимости. Организаторы смогут реализовать любую вашу мечту, фантазию, чтобы вы остались довольны результатом.
Компания https://torgstore.ru/ предлагает большой ассортимент торгового оборудования и мебели для магазинов, офисов, гостиниц, торговых залов в наличии и на заказ. Зайдите в каталог и ознакомьтесь с готовыми решениями или индивидуальными проектами.
best ed drug https://fintryides.com/
greengo shop
Забудьте о низких позициях в поиске! Наше SEO продвижение и оптимизация на заказ https://seosistemy.ru/ выведут ваш сайт в топ, увеличивая его видимость и привлекая потенциальных клиентов. Индивидуальный подход, глубокий анализ ключевых слов, качественное наполнение контентом — мы сделаем всё, чтобы ваш бизнес процветал.
пинап – это истинный азартный рай! Я не могу получить удовольствие от многообразному палитре развлечений и великодушным премиям. Моментальные возмещения и предупредительная служба поддержки сделали данное гемблинг-клуб избранным предпочтением. Рекомендую каждому желающему, кто бережет уровень и наслаждение от азарта!
На сайте https://profildoors57.ru вы сможете подобрать дверь в жилое или офисное помещение. Перед вами огромный выбор вариантов и самой разной цветовой гаммы. Регулярно на портале появляются новинки, с которыми необходимо ознакомиться и вам. На сайте имеются межкомнатные двери, а также системы открывания, качественная фурнитура и многое другое. Регулярно в магазине проходят акции, которые позволят существенно сэкономить. Перед вами огромный выбор вариантов – их здесь несколько сотен.
На сайте https://charovnica58.ru/ вы найдете качественную, эффективную и проверенную продукцию популярной компании «Асония». Есть возможность не только приобрести продукцию для сна, но и записаться на сеансы-тренинги, на которых вы узнаете много нового, полезного. Бренд «Чаровница» был создан еще в далеком 2007 году и заполучил огромное количество постоянных клиентов, которые рекомендуют продукцию своим друзьям. Прямо сейчас вы сможете заказать анатомический валик и многое другое по доступным ценам.
Автосервис «LEMON CAR» сохраняет лояльные цены на высококачественные услуги. Он собрал квалифицированную команду мастеров, готовых взять на себя все вопросы касаемо вашего авто. Специалисты применяют только современное оборудование. Ищете сто Анапа на карте? Lemon-car.ru – сайт, где вы узнаете стоимость и сроки. Клиенты автосервиса отмечают быстрое выполнение работ. Позвоните по телефону и вам дадут грамотную консультацию. Благодаря «LEMON CAR», ваш автомобиль будет ухоженно выглядеть и надежно работать в течение всего срока эксплуатации.
На сайте https://www.rcef.pro/ изучите всю необходимую информацию, которая касается третьего российско-китайского саммита, который проходил 21 апреля 2016 года. В отдельной колонке вы найдете итоги саммита за 2016 год, сможете изучить фотогалерею, итоги 2015 года. Также есть и итоги 2014 года, фотогалерея. Вся информация достоверная, свежая, актуальная и будет интересна всем. Также вы ознакомитесь и с организаторами мероприятия, партнерами, операторами. Обязательно почитайте новости, в которых освещаются все самые важные вопросы.
Pingback: how fast does bactrim work
100% одобрение займа: быстрый способ решения ваших финансовых нужд
100 процентный займ на карту без отказа https://zajm-100-procentov-odobreniya.ru/ .
Pingback: bactrim skin infection
Ed Drugs from India https://patriciaalanpaen.wordpress.com/
На сайте https://modelsescort.biz/ закажите роскошных, привлекательных стриптизерш на холостяцкую вечеринку. К вам придут дамы модельной внешности, которые обрадуют своими умениями, талантами. С такими заводными и веселыми девчонками время пройдет незабываемо и быстро. А самое важное, что они обязательно исполнят любую вашу прихоть. Есть возможность заказать одну или несколько девочек, у которых роскошная фигура и впечатляющие внешние данные. Получится пригласить их на любое количество времени.
fda approved ed treatment https://patriciaalanpaen.wordpress.com/
Vavada casino
Скачать музыку https://kissvk.me/ очень просто. Зайдите на сайт KissVK где представлены все самые свежие треки в mp3, коллекции, подборки, хоты, треки 2024 и треки прошлых лет, которые также можно слушать онлайн. Скачать бесплатно музыку в формате mp3 в один клик без регистрации на компьютер, телефон или планшет на нашем сайте.
דירות דיסקרטיות באשדוד
erectile dysfunction pills from india https://patriciaalanpaen.wordpress.com/
На сайте https://domdacha-stroy.ru/ вы сможете заказать строительство каркасных домов под ключ недорого. В разделе Наши работы вы сможете посмотреть реализованные проекты, а в каталоге посмотреть разнообразные варианты домов. Получите консультацию специалиста бесплатно.
Ed Drugs https://patriciaalanpaen.wordpress.com/
1хбет
ed pills for sale https://patriciaalanpaen.wordpress.com/
buy ed meds online https://patriciaalanpaen.wordpress.com/
Зайдите на сайт https://csgospn.pro/ где вы сможете выиграть скины CS GO,CS2. Мы предлагаем разнообразные режимы игры, включая игру на вещи Steam. Присоединяйтесь к нашему сообществу и испытайте удачу в CSGOSPN. Подробная информация на сайте.
На сайте https://sng-oil.com/ изучите все услуги, которые предоставляются Частным Образовательным Учреждением «Самарские Нефтегазовые Технологии». Здесь работают лучшие преподаватели, которые научат всему, что знают сами, повысят уровень квалификации, чтобы вы смогли лучше исполнять свою работу. У этого учебного заведения имеется лицензия, которая предоставляет право вести деятельность. По окончании выдаются документы. При небольших ценах предоставляются услуги высокого качества.
Безработным тоже доступны займы: как получить деньги без указания работы?
займы без работы на карту https://zajmy-bez-ukazaniya-raboty.ru/ .
best non prescribed ed pill https://patriciaalanpaen.wordpress.com/
ed drugs online canada https://patriciaalanpaen.wordpress.com/
what is the best non prescription erectile dysfunction https://patriciaalanpaen.wordpress.com/
Приветственный бонус Леон
ed drugs in india https://patriciaalanpaen.wordpress.com/
https://onlinecasinos.com.cy/
На сайте https://www.avtogalaktika.com/ вы сможете воспользоваться такой важной услугой, как заказ автомобилей из Европы и Америки. Есть машины как под заказ, так и в наличии. Есть возможность получить крупную сумму денег под залог автомобиля, займ под ПТС авто, под залог спецтехники, грузового автомобиля. Ознакомьтесь с теми автомобилями, которые имеются сейчас в продаже. Также представлены и технические характеристики, год выпуска, стоимость на данный момент. Для того чтобы воспользоваться услугами, заполните заявку на сайте.
generic ed drugs cost https://patriciaalanpaen.wordpress.com/
best ed drug for men https://patriciaalanpaen.wordpress.com/
Сайт https://hoster-solutions.net/ это возможность недорого купить прокси. Персональные прокси, пакетные прокси, мобильные прокси и многое другое всегда на сайте. Поддержка работает круглосуточно, а удобный личный кабинет дает возможность отслеживать все покупки. Все работает автоматически для удобства клиентов.
most popular ed medication https://patriciaalanpaen.wordpress.com/
Займ через Госуслуги на карту: быстро, удобно, с гарантией безопасности
займ госуслуги https://www.zajm-cherez-gosuslugi.ru .
Вывод из запоя профессионалами в области наркологии в Самаре
снятие похмельного синдрома https://www.vyvod-iz-zapoya163.ru/samara/lechenie-pohmelya .
new ed drugs https://patriciaalanpaen.wordpress.com/
Садовые качели Маркет – интернет-магазин, который гарантирует долговечность изделий и высокое качество. Здесь предлагают большой ассортимент садовых качелей на разный бюджет и на любой вкус. Создавайте собственный комфортный уголок в саду для отдыха. https://sadovye-kachelimarket.ru/ – сайт, где у вас есть возможность оставить заявку уже сейчас. Компетентные консультанты подберут модель, подходящую именно вам. Наши садовые качели обеспечивают максимальный комфорт благодаря мягким сиденьям и удобным спинкам. Закажите качели у нас, и мы доставим ваш заказ в короткие сроки.
best ed drugs for women https://patriciaalanpaen.wordpress.com/
ed drugs online from india https://patriciaalanpaen.wordpress.com/
http://windows10-th.ru/profile.php?u=mortalspark2
На сайте https://dahua-rus.ru/catalog/videonabludenie/ в огромном ассортименте представлены системы видеонаблюдения популярной марки «Dahua», которая считается одной из самых надежных. Здесь представлены различные варианты, которые различаются техническими характеристиками, модификацией, а также другими моментами. Имеются, в том числе, и антивандальные уличные варианты, которые потребуются всем хозяевам частных домов, коттеджей. Установлены привлекательные расценки, регулярно появляются новинки.
https://spbhousekupit.ru/
На сайте https://poulitcam.ru/afisha.html ознакомьтесь с расписанием всех экскурсий, которые организуются в Москве. А в 1.30 и до 2.30 проходят бесплатные экскурсии. Вы сможете записаться на них в ближайшее время и получить яркие, приятные эмоции. Вас точно не оставят равнодушным истории тех людей, которые ходили по этим местам, улицам, переулкам. Но для того, чтобы попасть на экскурсию, необходимо записаться на нее заранее. Однако есть и такие экскурсии, которые временно не проводятся.
https://yuzhnoukrainsk.pogovorim.su/viewtopic.php?id=9729#p25966
Наша команда профессиональных мастеров предоставлена выдвинуть вам современные подходы, которые не только снабдят надежную протекцию от заморозков, но и подарят вашему собственности элегантный вид.
Мы практикуем с новыми составами, подтверждая постоянный период работы и великолепные эффекты. Изолирование фасада – это не только экономия на огреве, но и заботливость о природной среде. Экономичные технические средства, которые мы внедряем, способствуют не только своему, но и сохранению природных богатств.
Самое ключевое: [url=https://ppu-prof.ru/]Прайс лист на утепление фасада[/url] у нас начинается всего от 1250 рублей за м²! Это бюджетное решение, которое преобразит ваш дом в подлинный уютный локал с минимальными затратами.
Наши труды – это не исключительно изолирование, это формирование поля, в где все компонент отражает ваш особенный моду. Мы учтем все все твои пожелания, чтобы осуществить ваш дом еще еще больше приятным и привлекательным.
Подробнее на [url=https://ppu-prof.ru/]http://ppu-prof.ru/[/url]
Не откладывайте заботу о своем помещении на потом! Обращайтесь к мастерам, и мы сделаем ваш домик не только тепличным, но и более элегантным. Заинтересовались? Подробнее о наших проектах вы можете узнать на веб-ресурсе. Добро пожаловать в универсум благополучия и качества.
https://spbkupitzhilie.ru/
трансфер новосибирск белокуриха http://transfer-novosibirsk-belokurikha.ru/
Привет любители музыки!
White Queen в очередной раз удивляет: сквозь трек ‘Дорога домой’ чувствуется искренность и неподдельная страсть.
Певица White Queen знает, как завоевать сердца слушателей. Ее новый трек “Дорога домой” наполнен искренностью, страстью и любовью к музыке.
Эта песня заставляет вас не только двигаться под ее звуки, но и чувствовать себя особенным.
Погрузитесь в мир музыки White Queen и прочувствуйте всю ее силу.
https://apple.co/3PtdqE6
[url=https://music.yandex.ru/album/29110942/track/121285337] дорога дорога домой [/url]
испанская песня танцевальная
английская музыка танцевальная
цыганская песня танцевальная
белорусская танцевальная песня
песня танцевальная кто поет
клубная песня танцевальная
арабская музыка танцевальная mp3
танцевальная клубная песня
восточная музыка танцевальная скачать
танцевальная музыка без слов скачать бесплатно
Удачи и хорошего вайба!
На сайте https://schmusic.ru/activity/ вы можете найти талантливых музыкантов разных жанров и стран. Здесь есть поп-исполнители, рок-группы, классики, джазмены и другие. У вас есть возможность выбрать музыку по своему настроению и вкусу, а также найти новых друзей. Портал предлагает удобный интерфейс, для того чтобы вы могли с легкостью отыскать то, что вас интересует. Если же вы хотите стать музыкантом, вам обязательно в этом помогут. Для этого на сайте разместите свою музыку и получите отзывы от пользователей.
Good morning!
п»ї
The customer service team at Globex Music is second to none. Artists can reach out with any questions or concerns, and the team is always happy to help.
This level of support is a huge benefit for musicians who may be new to the world of music distribution.
п»їhttps://distro.globexmusic.com/register.php?ref=ZU0VL
[url=https://distro.globexmusic.com/register.php?ref=ZU0VL] how to distribute a cover song [/url]
how to distribute a cover song
cover song license price
get mechanical license
where to get mechanical license for cover song
cover song licensing – get a mechanical license
song mechanical license
how to get a mechanical license for a cover song
how to get mechanical license for cover song
п»їcover song distribution
cover songs definition
Good luck and good vibe!
boat rentals cocoa beach fl https://boatrent.shop/
мобильная версия фреш казино
На сайте https://kzn-tour.ru подберите тур прямо сейчас. Для этого необходимо обозначить то, откуда вы планируете вылететь, а также дату вылета, количество ночей, туристов. Определитесь и с классом отеля, а также типом питания и другими нюансами. Ознакомьтесь с тем, какие туры вас ожидают в ближайшие месяцы. Изучите лучшие предложения, а также расценки на них. Прямо сейчас вы сможете устроить путешествие в Индию, Катар, Венесуэлу, Абхазию. Также вы сможете слетать на выходные в Италию или Тунис, чтобы получить приятные эмоции и впечатления.
Letting you send UNLIMITED emails every day, free, for the rest of your life.
And not only that…
Mail Mate will help you gain thousands of high-quality, high-converting leads to email, all in one powerful package.
Learn More https://www.youtube.com/watch?v=65PD1Wb-SPk
https://ekbflatkupit.ru/
На сайте https://deliveryplay.ru/arenda-metalloiskatelya воспользуйтесь такой важной и нужной услугой, как аренда металлоискателя. Специально для вас доступна оперативная доставка по Москве. Имеются устройства под любые запросы. Есть возможность воспользоваться арендой даже без залога. Предусмотрен комфортный самовывоз. Только в этой компании вы сможете воспользоваться поддержкой на протяжении всей аренды. При необходимости вы получите ответы на все вопросы или профессиональную консультацию.
кровать цена [url=krovati-moskva11.ru]krovati-moskva11.ru[/url] .
На сайте https://minivan.online закажите такси минивэн, которое можно вызвать в аэропорт, на свадьбу, воспользоваться детским креслом. Также получится воспользоваться и корпоративным транспортом, для поездки между городами. Многие выбирают минивэн, потому как он вместительный, в нем могут разместиться до 8 человек. На услуги предоставляются приемлемые цены, для вас есть профессиональный водитель, который аккуратно управляет транспортным средством. Вы сможете положить в багажник все необходимое, потому как он вместительный.
На сайте https://marniks.ru/stray-kids-catalog.html вы сможете приобрести функциональные, качественные и практичные ночники с интересной гравировкой, которая посвящена популярной группе «Stray Kids». Перед вами огромное количество интересных вариантов, которые украсят любое пространство, независимо от концепции. Любой ночник становится олицетворением изысканного и утонченного стиля. Для гравировки использовались исключительно инновационные и уникальные технологии. Купив такой ночник, вы погрузитесь в удивительный и яркий мир.
Leon iOS скачать
На сайте https://koroche-dealer.ru/ вы сможете ознакомиться с интересными материалами, которые касаются бизнеса. Перед вами только свежие, новые и актуальные статьи на данную тему. Они помогут лучше узнать все, что вас интересует. Материалы ответят на многочисленные вопросы, которые вас заботили. Вы узнаете про то, как правильно вести переговоры с определенной целью. Имеются и различные операционные практики, а также стратегические возможности. Кроме того, вы узнаете и о том, как получить лояльность клиентов в автосервисе.
На сайте https://yotech.ru/ почитайте актуальную, свежую информацию, которая касается высоких технологий, уникальных разработок и увлекательных новинок, которые будут интересны всем, кому знакома данная тема. На этом сайте в первую очередь появляются анонсы новых гаджетов, любопытные обзоры и прочая информация. Обязательно изучите раздел с наиболее популярным материалом. К примеру, вы узнаете о том, когда выйдет новый телефон, ознакомитесь с рейтингом лучших смартфонов. Также вы узнаете и о том, какой будет дизайн у нового смартфона.
Интернет магазин сантехники Sanbest https://sanbest.ru/ это возможность купить сантехнику в Москве по отличным ценам с доставкой. Ознакомьтесь с категориями и обратите внимание на уникальные цены в разделах и сможете купить душевую кабину в Москве, купить ванну в Москве, купить раковину в Москве по отличным ценам. Сантехника Москва – все лучшее собрано у нас на сайте!
http://klublady.ru/
требуются девушки на высокооплачиваемую работу
займ под залог авто воронеж
Доставка цветов в Харькове: когда хочется сделать сюрприз.
Заказать цветы на дом Харьков с быстрой обработкой заказа
На сайте https://xn—-7sbasd0ahefwoi.xn--p1ai вы сможете заказать такую востребованную услугу, как производство, размещение наружной рекламы. Все работы выполняются под ключ, а потому заказчику не нужно будет не за что переживать. К вашим услугам макеты, дизайн, производство, аренда и многое другое. Создаются вывески различной сложности. Для этого используется инновационное, проверенное и высокотехнологичное оборудование. Результат высокого качества и по привлекательной стоимости. Регулярно предоставляются акции, которые помогут сэкономить.
Зайдите на сайт https://leasingber.ru/ где вы сможете ознакомиться с условиями от разных банков и компаний по такой услуге как авто в лизинг для юридических лиц и ИП. Подайте заявку на лизинг онлайн и ее получат сразу все ведущие компании на рынке и предложат для вас лучшие условия.
На сайте https://vavadamow.com поиграйте в интересные, увлекательные игры, которые подарят положительные эмоции от азарта. Перед вами около 1000 игр, которые представлены лучшими мировыми провайдерами. Именно поэтому вы точно сможете рассчитывать на реальный выигрыш. На сайте вы найдете классические слоты, а также живые игры с дилерами. Имеется щедрая система бонусов, включая приветственные. Для того чтобы защитить данные игроков, используются особые цифровые коды. Получить доступ ко всем привилегиям вы сможете, пройдя регистрацию.
На сайте http://fotolombard.ru обратитесь за займом. В компанию вы сможете сдать технику любого вида. С ее полным перечнем ознакомьтесь на сайте. Если и вам необходимы деньги срочно, то скорей обращайтесь за помощью сюда. Здесь весь процесс является быстрым, официальным, а процент максимально низкий. Сотрудничество с компанией надежное, вы сможете на нее рассчитывать. Всего 15 минут понадобятся на оформление, после чего вы сразу получите деньги. Сумма будет намного больше рыночной. Укажите в заявке все особенности техники.
У нас вы посчитаете все необходимое – от качественных продуктов до сверхтехнологичных услуг. Наш каталог разнообразен, чтобы ублаготворить любые потребности.
Почему стоит посетить наш сайт?
Широкий ассортимент продукции: У нас вы обнаружите последние инновации и технологические достижения в наших товарах и предложениях.
Инновации и качество: Мы гордимся тем, собственно что предлагаем лишь только лучшие решения.
Специальные предложения: Посещение нашего сайта – это вероятность первыми распознавать о особых услугах, промоакциях и скидках.
Экспертные советы и статьи: Мы делимся с вами экспертными советами, статьями и советами по использованию наших товаров.
Пользовательский опыт: Наш веб-сайт разработан с учетом вашего комфорта. Интуитивно понятный интерфейс, стремительная навигация и простота в использовании обеспечивают милый онлайн-опыт.
собственно что вас ожидает на нашем веб-сайте?
Разделы [url=https://forum.bocu.ro/viewtopic.php?p=80796]https://forum.bocu.ro/viewtopic.php?p=80796[/url]
:
Каталог продуктов: Ознакомьтесь с нашим различным ассортиментом. От товаров ежедневного спроса до оригинальных и эксклюзивных предложений – у нас все есть!
Услуги: Узнайте о наших сверхтехнологичных услугах, предоставляемых профессиональными специалистами.
Блог и заметки: Погрузитесь в мир экспертных советов и интересных материалов. Мы делимся информацией, кот-ая поможет для вас сделать верный выбор и получить максимальную пользу от наших продуктов.
Специальные предложения: Будьте в курсе акций и бонусов. У нас регулярно ведутся специальные события, приуроченные к разным событиям.
Обратная связь: Мы ценим ваше суждение. В разделе “Обратная связь” вы можете оставить комментарии, отзывы и задать вопросы. Ваше мнение принципиально для нас!
Как воспользоваться сайтом?
Выберите категорию: Перейдите в подходящий раздел каталога или предложений и изберите интересующий вас товар или предложение.
Изучите контент: Ознакомьтесь с увлекательными заметками и блогом, чтобы получить дополнительную информацию о продукции и услугах.
Добавьте в корзину: Для покупок просто добавьте товар в корзину и следуйте наставлениям для дизайна заказа.
Подпишитесь на анонсы: Будьте в курсе всех событий, подписавшись на нашу рассылку. Получайте первыми информацию о новинках и промоакциях.
Оставьте отзыв: Поделитесь своим навыком с нашими товарами и услугами. Ваши отзывы посодействуют другим клиентам сделать верный выбор.
В заточение:
Мы приглашаем вас в наш виртуальный мир. Мы рвемся предоставить вам не столько продукцию и предложения высокого качества, но и неповторимый навык покупок. Посетите наш вебсайт, и вы удостоверьтесь, что у нас есть все для вашего комфорта и ублажения. Приятных для вас открытий на страницах нашего веб-ресурса!
Рострубодеталь https://rostrubodetal.ru/ – завод по производству труб и деталей трубопровода с доставкой по России и СНГ. Зайдите на сайт и ознакомьтесь с каталогом бесшовных, точеных и сварных деталей трубопровода, фланцевой продукции. Подробнее на сайте.
http://diplombiolog.ru/
купить сплит систему в интернет магазине недорого [url=http://www.split-sistema11.ru/]http://www.split-sistema11.ru/[/url] .
На сайте https://paints-market.ru/ в большом ассортименте представлены профессиональные краски: по металлу, на водной основе и многое другое. Для того чтобы найти что-то определенное, необходимо ввести в строку название, а затем система подберет то, что нужно. Регулярно на портале появляются новинки, которые потребуются и вам. Постоянно проходят акции, действуют скидки. При необходимости воспользуйтесь помощью менеджера. Постоянно выставляются новые товары со скидкой. Почитайте отзывы клиентов, чтобы узнать, что говорят о магазине.
http://diplombuhgalter.ru/
https://yuzhnoukrainsk.pogovorim.su/viewtopic.php?id=10395#p27527
На сайте https://hozpak777.ru/ приобретите одноразовую качественную посуду, созданную из практичных и надежных материалов, либо прочные пакеты, которые понадобятся вам для фасовки продуктов. Вся продукция реализуется оптом, а цены могут изменяться, поэтому реальные и актуальные расценки необходимо уточнить у менеджера заранее. Доставка осуществляется в минимальные сроки и наиболее комфортным способом. Вся продукция отличается высоким качеством, она прочная, надежная и функциональная.
В нашем кинотеатре https://hdrezka.uno смотреть фильмы и сериалы в хорошем HD-качестве можно смотреть с любого устройства, имеющего доступ в интернет. Наслаждайся кино или телесериалами в любом месте с планшета, смартфона под управлением iOS или Android.
https://kursovyebiolog.ru
https://kursovyebuhgalter.ru
Кадровое агентство Карьера Старт https://kariera-start.ru/ поможет вам в поиске высокооплачиваемых вакансий на курортах Израиля. Мы обеспечиваем полное сопровождение на весь период вашего пребывания в стране. Зайдите на сайт и посмотрите вакансии и наши предложения.
Надёжный стабильный хостинг от https://hoster.solutions/ по лучшей цене. Широкий выбор серверов и тарифных планов, гибкая система скидок. Круглосуточная высококвалифицированная поддержка. Партнерская программа.
На сайте https://ayaks-trening.ru/ закажите обратный звонок для того, чтобы узнать больше об учебно-тренинговом центре «АЯКС». Вы сможете повысить личную эффективность, решить все важные бизнес-задачи. В компании предоставляется полный и исчерпывающий комплекс услуг, в том числе, проводятся семинары и тренинги, консалтинг, тимбилдинг, устраиваются выездные программы, проводится индивидуальное консультирование. Компания находится на рынке более 15 лет, а потому ей можно доверять. Все тренеры обладают высокой квалификацией.
На сайте https://faktura-decor.ru/ вы найдете венецианскую штукатурку, краски, фактурные покрытия, необходимый профессиональный инструмент, огромный ассортимент материалов для дерева. Покупатели чаще всего выбирают продукцию для фасада, интерьера, дерева и многое другое. Перед вами весь ассортимент, с которым вы сможете ознакомиться прямо сейчас и подобрать то, что нужно. А для того, чтобы получить полное представление о товаре, необходимо ознакомиться с техническими характеристиками, а также ценами.
Visit the website https://wishes.pics/ where you will find a huge number of pictures with wishes for every morning or every day of the week or weekend. Please your loved one with a picture that will set him in the mood for the whole day!
На сайте https://vavadagow.com находится огромное количество игр, в которые получится сыграть в любое время: на компьютере или смартфоне. Всех пользователей привлекает не только большой выбор игр, но и щедрая бонусная система. Выигрыш выводится очень быстро, а сама компания работает на честных, прозрачных условиях. Клиенты получают возможность постоянно принимать участие в акциях, которые дают любопытные бонусы. Для получения всех привилегий следует пройти регистрацию. Каждую неделю зачисляется кэшбек от затраченной игроком суммы.
https://davidwej.com/soglasno-zakony-ru/
https://dev.to/extralabs/how-to-make-money-from-email-newsletters-tips-for-beginners-5doo
https://blockchainreporter.net/decoding-ach-crypto-and-its-role-in-the-alchemy-pay-ecosystem/
https://zagorie.mybb.ru/viewtopic.php?id=8680#p22210
На сайте https://penetron-ug.ru/ оставьте заявку для того, чтобы приобрести комплексную защиту бетона: Пенетрон, Пенекрит, Пенеплаг, Ватерплаг, Пенетрон Адмикс и многое другое. Вас ожидает отличное качество, которое проверено с 1991 года. Вся продукция оригинальная, сертифицированная, а потому подарит необходимый результат. Может применяться в любых местах и помещениях, в которых имеется бетон, включая подвалы, жилые дома, атомные электростанции. На всю продукцию установлены доступные цены.
советы по строительству
https://extralabs.github.io/
крупные криптовалютные биржи
Standard Price for VIP- membership for 1 Week VIP Membership is 0.0014 BTC, You will do send payment to BTC address 1KEY1iKrdLQCUMFMeK4FEZXiedDris7uGd
Discounted price may be different from 0.00075 to 0.00138 BTC,
that is why follow to all announces published in our Public channel!
trading signals
Pingback: amoxicillin and birth control how long to wait
https://zadachbiolog.ru/
На странице https://eduniko.ru/download-1xbet/ вы сможете скачать 1xBet на андроид, а также узнать полезную информацию о том, как скачать и установить, как обновить до последней версии, о бонусах и промокодах в приложении, как начать делать ставки и многое другое.
לכל אחד שמחפש חוויה הכי מפנקת! אמנות הפינוק האירוטי של נערות שמחכות לכם ב דורשת מיומנות, רגישות והבנה מעמיקה של האזורים אינטימית הכי עמוקה! בהזמנה לבתי מלון בילוי דיסקרטי מפנק במלון או בביתך הפרטי עם מספק שער להנאה אירוטית חושנית ותשוקה מעבר נערות ליווי בחיפה
Привет. С радостью информируем о запуске обновленного мобильного приложения букмекерской конторы Олимп! [url=https://best-olimp-app.ru/]Скачать БК Олимп[/url] возможно сегодня! Приложение теперь еще удобнее и быстрее, открывая доступ к большому ассортименту ставок на спорт с мобильных устройств. С новейшим обновлением вы получаете усовершенствованные функции управления вашим счетом, переработанный дизайн для еще более интуитивного пользования, а также повышенную скорость работы. Подключайтесь к довольным пользователям и делайте свои ставки с наслаждением и комфортом в любое время и в любом месте. Скачайте последнюю версию приложения букмекерской конторы сегодня и играйте вместе с Олимп!
https://t.me/crypto_signals_binance_pump/24498/ Standard Price for VIP- membership for 1 Week VIP Membership is 0.0014 BTC, You will do send payment to BTC address 1KEY1iKrdLQCUMFMeK4FEZXiedDris7uGd Discounted price may be different from 0.00075 to 0.00138 BTC, that is why follow to all announces published in our Public channel!
Pingback: cozaar mechanism of action
Pingback: cbd and citalopram
Приветствую. С радостью сообщаем о выходе улучшенного софта БК Олимп! [url=https://best-olimp-app.ru/]Букмекерская контора Олимп на андроид[/url] уже сегодня! Приложение стало еще комфортнее и функциональнее, открывая доступ к большому ассортименту ставок на спорт с мобильных устройств. С новейшим обновлением вы получаете расширенные функции управления вашим счетом, обновленный дизайн для еще более интуитивного использования, а также повышенную скорость работы. Присоединяйтесь к довольным пользователям и совершайте свои ставки с наслаждением и комфортом в любое время и в любом месте. Загрузите актуальную версию приложения букмекерской конторы сегодня и ставьте вместе с Олимп!
https://zadachbuhgalter.ru
Женская одежда от https://zinnaiz.kz/ – это дизайнерская женская одежда – платья, костюмы, брюки, блузы. Зайдите на сайт и вы увидите уникальные вещи высокого качества. Ознакомьтесь с каталогом и адресами магазинов. Присутствует и раздел товаров со скидками. Подробнее на сайте.
מגוון אופציות לבחירה. בחר מודעות של משרדים ששולחים בחורות מאומנות ושומרים על סודיות הלקוח. קריאת ביקורות וחיפוש המלצות יכולים האירוטיות שלך ולאמץ את הרצונות החושניים. עם מוסיקה רכה, ריחות מרגיעים ותאורה עמומה יוצרים אווירה של רוגע ואינטימיות תמיד פתוחות דירות דיסקרטיות באשדוד
Pingback: augmentin doses for sinusitis
Клиника СВЕТОДАР оказывает широкий спектр офтальмологических услуг и заботится о потребностях пациентов. Стремимся учесть их пожелания, чтобы лечение было комфортным и действенным. Медицинский персонал центра офтальмохирургии состоит из специалистов высокой квалификации, заботу о своих глазах можете им доверить. https://sp.svetodar.pro/ – сайт, где вы сможете получить нужную информацию о клинике. Здесь можно ознакомиться со списком предоставляемых услуг и отзывами пациентов. Мы регулярно работаем над повышением сервисного качества.
https://otchetbiolog.ru/
AdisPrint https://adisprint.ru/ это огромный каталог расходных материалов для печати с доставкой, такие как СНПЧ, ПЗК, чернила, картриджи, тонер, бумага, промывочная жидкость, программаторы, пластик для 3D печати и другие расходные материалы для принтеров и многое другое. Подробнее на сайте.
Pingback: how long for effexor to work
https://otchetbuhgalter.ru/
На сайте https://sweetbonanzag.com/ вы сможете узнать всю необходимую информацию об онлайн-слоте SWEET BONANZA, который пользуется огромной популярностью. У него стильный и привлекательный дизайн, интересное оформление. При этом вы гарантированно получите выигрыш, который будет выведен в ближайшее время. Игроки смогут воспользоваться бонусными спинами, которые увеличивают выигрыш. Доступны и другие интересные опции и привилегии. Испытайте возможности слота в бесплатном режиме.
מחכות לך כמה נערות מהממות, הן זמינות בשבילך ביום ובלילה גם בסופי שבוע, תרגיש חופשי להתקשר אליהן ולשאול פרטים על השירות שלהן. להדריך אותך לקראת חוויה בטוחה ומהנה. התקשר או שלח הודעת ווצאפ למספרי טלפון שמופיעים בכל מודעה כדי לברר פרטים קטנים וכל מה שחשוב נערות ליווי בתל אביב
Восторгаемся возможностью анонсировать новейшее обновление приложения от БК Олимп для Android! Ваше взаимодействие со ставками на спорт станет еще более увлекательным благодаря усовершенствованному интерфейсу и оптимизированной работе программы. [url=https://best-olimpbet-apk.ru/]Olimp bet на телефон[/url] возможно сегодня! С последней версией приложения вы получите неограниченный доступ к разнообразию спортивных событий прямо с вашего мобильного устройства. Ожидайте расширенные возможности для управления счетом, передовой дизайн для интуитивного пользования и значительное улучшение скорости приложения. Станьте частью довольных клиентов БК Олимп и наслаждайтесь ставкам где угодно и когда угодно. Установите последнюю версию приложения без промедления и начните выигрывать вместе с Олимп!
Pingback: diltiazem dose for afib with rvr
Восторгаемся возможностью анонсировать новейшее обновление приложения от БК Олимп для Android! Ваше умение и опыт со ставками на спорт будет еще более увлекательным благодаря обновленному интерфейсу и ускоренной работе программы. [url=https://best-olimpbet-apk.ru/]Скачать приложение Olimp[/url] доступно сегодня! С последней версией приложения вы получите прямой доступ к множеству спортивных событий прямо с вашего мобильного устройства. Ожидайте расширенные возможности для управления счетом, современный дизайн для интуитивного пользования и значительное улучшение скорости приложения. Станьте частью довольных клиентов БК Олимп и наслаждайтесь ставкам где угодно и когда угодно. Установите последнюю версию приложения уже сейчас и начните выигрывать вместе с Олимп!
Pingback: orlistat vs contrave
Pingback: flomax febbre
Сайт https://moscredit.org/ это автоломбард в Москве. Зайдите на портал и вы сможете рассчитать сумму, которую получите за свой автомобиль, а также ознакомиться с условиями. Вы можете использовать автомобиль в качестве залога и моментально получить деньги на карту в день обращения.
С энтузиазмом сообщаем о запуске переработанного мобильного приложения БК Олимп для Android устройств! Этот шаг значительно улучшает ваше прежнее взаимодействие с ставками, делая его проще и эффективным. [url=https://skachat-olimp-app.ru/]Скачать БК Олимп[/url] уже сегодня! В новой версии приложения пользователи обретут непосредственный доступ к широкому спектру спортивных соревнований через свой смартфон. Оптимизированное управление аккаунтом, новаторский дизайн для легкого навигации и значительное ускорение работы приложения – всё это ждет вас. Станьте одним из счастливых пользователей и испытывайте радость от ставок в любом уголке мира и всегда. Загрузите обновление приложения БК Олимп сегодня и откройте для себя новый уровень игры!
Pingback: diclofenac sodium topical gel 1 reviews
С энтузиазмом сообщаем о дебюте нового мобильного приложения БК Олимп для Android устройств! Этот релиз значительно улучшает ваше прежнее взаимодействие с ставками, делая его более интуитивным и быстрым. [url=https://skachat-olimp-app.ru/]Контора Олимп на андроид[/url] доступное для игроков! В новой версии приложения пользователи обретут непосредственный доступ к широкому спектру спортивных соревнований через свой смартфон. Оптимизированное управление аккаунтом, новаторский дизайн для легкого навигации и значительное ускорение работы приложения – всё это ждет вас. Станьте одним из удовлетворенных пользователей и наслаждайтесь от ставок где угодно и в любой момент. Загрузите обновление приложения БК Олимп сегодня и откройте для себя новый уровень ставок!
Свартехкомлект предлагает сварочные материалы и оборудование по наилучшим ценам. У нас имеются в наличии выпрямители, инверторы, трансформаторы, горелки, генераторы, реостаты, резаки и многое другое. Квалифицированные специалисты помогут вам с подбором, они быстро обрабатывают заявки. https://www.svartk.ru/ – сайт, где предложен для сварки большой выбор расходных материалов. Гарантируется доставка в короткие сроки. Решив приобрести у нас сварочное оборудование, можете быть уверены в результативности осуществляемых работ.
Посетите сайт АудиоВидеоСистемы https://audioprofi.ru/ – компанию, которая занимается оснащением световым и звуковым оборудованием и является ведущим разработчиком и интегратором мультимедиа решений для различных площадок. Наши компетенции позволяют решать любые задачи. Подробнее на сайте.
Pingback: ezetimibe solid dispersions
Pingback: what are ddavp tablets
https://resheniezadachfizika.ru/
Pingback: how long does it take for flexeril to work
С огромной радостью разделяем новостью о релизе обновленной версии мобильного приложения от БК Олимп для Android! Это обновление изменит ваш подход к ставкам на спорт, делая процесс более гладким и эффективным. [url=https://olimpbet-apk.ru/]Скачать БК Олимп для андроид[/url] и вы получите доступ без труда к широкому ассортименту спортивных мероприятий, доступных для ставок прямо с вашего мобильного. Усовершенствованные функции управления профилем, революционный дизайн для легкости использования и ускорение скорости приложения обещают непревзойденный опыт. Становитесь частью сообществу довольных клиентов и делайте ставки с удовольствием где бы вы ни находились, в любое время. Установите последнюю версию приложения БК Олимп немедленно и выходите на новый уровень ставок с комфортом и стилем!
https://kursovyemarketing.ru/
С великим удовольствием разделяем новостью о выпуске новаторской версии мобильного приложения от БК Олимп для Android! Это обновление изменит ваш подход к ставкам на спорт, делая процесс более гладким и эффективным. [url=https://olimpbet-apk.ru/]Бесплатно скачать Олимп бет на андроид[/url] и вы получите доступ без труда к огромному выбору спортивных мероприятий, открытых для ставок прямо с вашего мобильного. Усовершенствованные функции управления профилем, революционный дизайн для упрощения навигации и значительное повышение скорости приложения обещают непревзойденный опыт. Присоединяйтесь к сообществу довольных клиентов и наслаждайтесь ставками где бы вы ни находились, в любое время. Установите последнюю версию приложения БК Олимп уже сейчас и выходите на новый уровень игры с комфортом и стилем!
http://avicenna-s.ru/
Зайдите на сайт практикующего психолога-психотерапевта https://emysakov.online/ Евгения Мысакова где вы сможете получить эффективную психологическую помощь даже в самых сложных ситуациях. Опыт консультирования с 2010 года позволяет решить практически любую проблему и помочь человеку справится с ней. Подробнее на сайте.
Услуга демонтажа старых частных домов и вывоза мусора в Москве и Подмосковье. Наши специалисты бесплатно выезжают на объект для консультации и оценки объема работ. Мы предлагаем услуги на сайте https://orenvito.ru по доступным ценам и гарантируем качественное выполнение всех работ.
Для получения более подробной информации и рассчета стоимости наших услуг, вы можете связаться с нами по телефону или заполнить форму заявки на нашем сайте.
Pingback: what is depakote used to treat
https://1ecenter.ru
На сайте https://1-3.su вы найдете былины, сказки, колыбельные, загадки, а также стихи. Здесь представлены самые разные сказки, которые можно прочитать на ночь и детям. Имеются произведения и для самых маленьких, редкие сказки, которые подходят для прочтения перед сном. Также представлены и колыбельные, которые помогут получить хорошее настроение. В этой библиотеке огромное количество самых интересных произведений. Все они от высококлассных авторов. Можно читать книги, независимо от того, где вы находитесь.
Желаете по недорогой цене купить чемодан на колесах? FEELWAY вам в этом поможет. Предоставляемые нами чемоданы изготовлены качественно, у них крепкие колеса и хорошие молнии, они создают настроение отпуска. Легко настраивается кодовый замок. Приобретением вы точно останетесь довольны. https://feelway.ru/ – сайт, где можно узнать, из чего выполнен чемодан. Также здесь вы можете проверить подлинность товара. Просто введите ваш email, код изделия и нажмите на специальную кнопку «Отправить». Обращайтесь к нам, проконсультируем!
לרצונותיכם. מציעות לכולם מגוון רחב של שירותים אירוטיים הניתנים על ידי נערות מדהימות והכי מקצועיות. צאו עכשיו למסע של הנאה פרטיות המציעות לגברים חוויות מיוחדות, ייחודיות והכי אינטימיות. ביקור במקומות כמו זה תמיד פינוק הכי לוהט ואיכותי לכל טעם וכיס. דירות דיסקרטיות באשקלון
Завод весового оборудования ООО ЗВО https://uzvo.ru/ – это производство и продажа весов в большом ассортименте, таких как: автомобильные весы, железнодорожные вагонные весы, весовые дозаторы для фасовки, конвейерные весы, платформенные весы, весы для животных, бункерные весы, весы на погрузчик и многое другое. Подробнее на сайте.
Услуга демонтажа старых частных домов и вывоза мусора в Москве и Подмосковье от нашей компании. Мы предлагаем демонтаж и вывоз мусора в указанном регионе по доступным ценам. Наша команда https://hoteltramontano.ru гарантирует выполнение услуги в течение 24 часов после заказа. Мы бесплатно оцениваем объект и консультируем клиентов. Узнать подробности и рассчитать стоимость можно по телефону или на нашем сайте.
На сайте https://www.dokonlin.online/ вы сможете смотреть Шоу и Телепередачи онлайн. Воспользуйтесь поиском или смотрите последние поступления, ТОП по просмотрам или ТОП по рейтингу. Один из самых крупных и ежедневно пополняемый видео каталог новых телепередач, документальных фильмов, шоу, телешоу, сериалов.
ידועות באווירה אינטימית ונעימה שלהן ובנערות ליווי הכי יפות שמזמינות אותך לגן עדן של הנאה בלתי פוסקת! לבחירתך יש מגוון המציעות סקסיות ומיומנות באמנות המגע האירוטי מבטיחות שהחוויה תהיה מהנה ומקצועית כאחד. בנוסף, הן מבינות היטב את החשיבות של שביעות רצון דירות דיסקרטיות בבאר שבע
Компания «VIVAT» успешно занимается производством и продажей спецодежды в Иваново. Грантируются приемлемые цены, быстрая доставка и высокое качество продукции. Сотрудники все без исключения внимательные, обслуживание хорошее. Ищете спецодежда на заказ? Vivat-iv.ru – сайт, где вы узнаете, как правильно подобрать костюм для сварщика. Также здесь возможно в любое удобное время ознакомиться с условиями оплаты. Заказывать у нас очень удобно, можете убедиться в этом сами. Ждем вашего звонка, обращайтесь!
Эффективность многофакторной аутентификации в современном мире
приложение multifactor [url=https://www.mnogofaktornaya-autentifikaciya.ru]kz vpn[/url] .
Провести время интересно можно всегда на сайте Smotret.net, именно здесь представлены достойные сериалы. Удобно расположитесь на своем диване или же кресле и приступите к просмотру. https://smotret.net/ – сайт, который предлагает расслабиться и отдохнуть, тут есть широкая коллекция отменных и качественных сериалов. Смотреть их можно в любой удобный момент. Гарантируем, что вас ждет множество занимательных историй. Также предлагается отличная возможность давать свои комментарии к сериалам. Приятного вам времяпрепровождения!
https://www.wakewiki.de/index.php?title=Benutzer:SantoHopman6
https://vostochniy.express/2023/12/04/kalifornijskij-sud-vynes-reshenie-v-polzu-rossijskogo-oligarha-stanislava-kondrashova-i-ego-kompanii-telf-ag-soglasno-kotoromu-blokirovke-podlezhat-ukrainskie-novostnye-veb-sajty/
[url=http://shypo.gsdt.kr/bbs/board.php?bo_table=inquiry&wr_id=573141]asdfghjkex[/url] e574_76
Привет всем!
https://i4car.net/category/avtonovosti/ – ваш путеводитель в мире автомобилей. Здесь вы найдете все от обзоров и тест-драйвов до советов по выбору и уходу за автомобилем.
[url=https://i4car.net/]i4car.net[/url]
i4car.net
дтп сегодня видео
Удачи и хорошей дороги!
Услуга демонтажа старых частных домов и вывоза мусора в Москве и Подмосковье от нашей компании. Мы предлагаем демонтаж и вывоз мусора в указанном регионе по доступным ценам. Наша команда гарантирует выполнение услуги стоимость сноса дома в течение 24 часов после заказа. Мы бесплатно оцениваем объект и консультируем клиентов.
Зайдите на сайте https://xn—–7kcabhfrga0adaatkdliqn0bthojetpj.xn--p1ai и вы сможете ознакомиться с таким полезным инструментом как анализ и аналитика ваших продаж на ведущих маркетплейсах. Ежедневные отчеты с ключевыми показателями продаж позволят вам проводить глубокую аналитику для достижения наилучшего результата. Подробнее на сайте.
https://acompromat.net/persons/kondrashov_stanislav/
[url=https://www.amatagroup.ru/forum/messages/forum1/topic59/message307760/?result=reply#message307760]asdfdghfgeref[/url] 900f0ec
https://dzen.ru/a/ZW_DJ7fFXy5opNbD
[url=http://shjrp.gsdt.kr/bbs/board.php?bo_table=inquiry&wr_id=573306]fccwccwcw[/url] 50d0590
волчонок все серии
https://na-dache.pro
הלקוח ומספקות שירות מותאם אישית המבוסס על העדפות ורצונות אישיים של כל אחד. מזמינות אותך עכשיו לביקור כדי לבנות אנרגיה מינית שלך האירוטית הלא נגמרת אף פעם. זו עיר שלא ישנה לעולם, שבה אנשים מכל תחומי החיים מתאחדים כדי לחוות את הנאות החושניות ביותר בעולם. כאן דירות דיסקרטיות בתל אביב
Популярная компания FARBWOOD предлагает купить продукцию из лиственницы в Минске по привлекательной стоимости. В работе своей используем только современное оборудование, гарантируем выгодные скидки, широкий ассортимент изделий, быструю доставку и высокое качество. farbwood.by – https://farbwood.by/сайт, где имеется возможность детальнее посмотреть условия доставки и оплаты. Также здесь есть каталог, контактная информация и галерея. Позвоните нам, мы проконсультируем по каждому товару либо по услуге.
1win казино
https://orda.kz/kazhrom-zakljuchil-mnogomilliardnuju-sdelku-so-shvejcarskoj-kompaniej-telf-ag-svjazannoj-s-rossiej-375968/
[url=https://dobrohim.ru/product/batsflakon50ml/]rfvddfyvyyv[/url] 900f0ec
Посетите сайт https://l2-top.ru/ который предлагает вашему вниманию анонсы серверов LineAge 2, а также посмотреть сервера ожидаемые к открытию или которые открылись раньше. Вы можете добавить и свой сервер. У нас вы легко сможете подобрать себе подходящий интересный сервер и начать играть в любимую Линейдж 2.
https://lenta.ua/ru/kondrashov-stanislav-dmitrievich-i-telf-ag-kak-voskresili-denisa-voronenkova-22358/
[url=http://www.2qh.net/club/forum/messages/forum1/topic170/message3397526/?result=reply#message3397526]asdfghjkl;[/url] 199926e
Заказать кейтеринг в Москве на сайте https://setbox.ru/ от SetBox это доставка готовых блюд от кейтеринга в коробках на дом и в офис. Зайдите на сайт и ознакомьтесь с широким ассортиментом блюд, ценами, условиями доставки. Заказ оформляется за пару кликов, а доставка занимает совсем немного времени. Подробнее на сайте.
Pingback: aripiprazole mechanism of action
Pingback: how long does amitriptyline make you sleepy
Хотите смотреть русские сериалы онлайн абсолютно бесплатно? Serialry – это то, что вам нужно. Здесь имеются мелодрамы, боевики, приключения, триллеры и многое другое. Вы сможете быстро сориентироваться в выборе видеопродукции. Ищете смотреть бесплатно все сериалы мира онлайн? Serialry.online – известный сайт, где кино представлено в безупречном качестве. Команда сервиса подготовила интригующие и увлекательные фильмы. Смотреть их можно в любое удобное для вас время. Можете оставить собственный комментарий о сериале. Уверены, что вам понравится наш ресурс.
Pingback: allopurinol dosage for gout
Специалисты известной клиники эстетической косметологии «REMEDY LAB» постоянно проходят курсы повышения квалификации. У них есть современные аппараты, все используемые средства прошли сертификацию. Стоимость на услуги доступна людям с различным уровнем дохода. https://remedylab.ru/apparatnaya-kosmetologiya/ – сайт, где можно в любое удобное время записаться на прием. Гарантируем индивидуальный подход, делаем все, чтобы клиенты чувствовали себя уютно. Сохраним вашу молодость и красоту, обращайтесь!
Pingback: how to safely wean off daily aspirin
https://wiggles.ruka.at/wiki/index.php/Ek-x.ru
http://www.diywiki.org/index.php/Fotosalon
https://elearnportal.science/wiki/User:NataliaChen119
Клининговая компания в Санкт-Петербурге https://proffcleaning.ru/ ПРОФ Клининг СПб это услуги профессиональная уборка квартир, офисов, домов и коттеджей, складов, клининг торговых и бизнес центров, уборка промышленных помещений и территорий по отличным ценам с профессиональным персоналом. Подробнее не сайте.
seo продвижение сайта цена в москве
Смотреть онлайн https://www.kinodraiv.pro/ ток-шоу, телешоу, фильмы и сериалы, документальные фильмы онлайн в хорошем качестве, бесплатно без регистрации. Огромная коллекция, которая пополняется ежедневно. Выберите категорию или смотрите ТОП. Все самое новое и свежее, а также коллекции за прошлые годы.
https://adzan.online/
Желаете купить по доступной стоимости подшипники с быстрой доставкой? Мы готовы решить этот вопрос! Заманчивые цены и большой ассортимент позволяют обеспечивать для сотрудничества комфортные условия. Весь товар прошел сертификацию. https://vse-podshipniki.ru/ – сайт, где можете сделать запрос. Мы гордимся отменным сервисом, который предоставляем нашим клиентам. Гарантируем полную удовлетворенность при приобретении у нас. Если вам требуется помощь в выборе подшипников, звоните нам уже сейчас! Мы окажем вам грамотную помощь.
Посетите сайт https://ru.binarybrokers.online/ и вы сможете ознакомиться с рейтингами бинарных опционов от Binary Brokers. Таблица брокеров 2024 позволяет узнать все плюсы и минусы конкретного брокера, а также все преимущества работы с ним. На сайте вы также сможете получить ответы на часто задаваемые вопросы о бинарных опционах.
сколько стоит механизированная штукатурка [url=http://www.mekhanizirovannaya-shtukaturka11.ru]http://www.mekhanizirovannaya-shtukaturka11.ru[/url] .
Здравствуйте автомобилисты!
https://dtp.vn.ua/category/video/ — ваш надежный источник новостей о ДТП в Виннице. Мы освещаем самые важные события, связанные с дорожными авариями, предоставляя аудитории не только новости, но и обучающие материалы для предотвращения будущих инцидентов на дороге.
[url=https://dtp.vn.ua/category/avto-news/]Авто новости Винницы [/url]
ДТП Винница видео
Новости Винницы
Удачи и хорошей дороги!
ингаляционнная медтехника
Лучшие картинки различных тематик https://stilno.site
DAHUA предоставляет исключительно качественную продукцию. Здесь вы сможете найти переговорные устройства, видеокамеры, домофоны и другое. О доступный ценах, даже и не нужно говорить, вы их увидите, открыв каталог. Сравните с другими предложениями. На все товары распространяется гарантия. Ищете видеорегистратор dahua? Dahua-rus.ru – сайт, где представлены часто задаваемые вопросы и ответы на них. Мы доросли до успешного интернет-магазина с сотнями довольных клиентов. Вы всегда можете получить профессиональную консультацию по телефону. Звоните, обязательно вам поможем!
casino bonuses
Хакер с достаточным опытом, успешно предлагает услуги взлома различной сложности. Он имеет много выполненных проектов. Специалист выходит на новейший уровень набора клиентов, после чего закрывает тему и уходит в приватную работу. Пока его услуги открыты ими может любой воспользоваться. https://xakervip.com/topic/282/page/10/ – сайт, где есть возможность детальнее посмотреть все услуги. Опытный хакер известен в сети, он берется за любые проекты и не видит никаких препятствий. Вы можете обратиться к нему в удобное для вас время. Грамотный специалист вас проконсультирует.
Go to https://doctor-nurse.com/ and check out the resource information on medical abortion and abortion pills. Read the blog and get useful information about Plan C Connect.
https://pro-dachnikov.com
хороший гражданский юрист
На сайте https://ruchkin.ru/ закажите сувенирные ручки или авторучки в огромном ассортименте и по доступным ценам. Предприятие производит ручки уже в течение длительного времени, считается лидером в данной сфере, а потому ему точно можно доверять. Вся продукция создается на инновационном, качественном оборудовании, что исключает возможность брака. Регулярно публикуются новости, которые помогут узнать больше информации. Установлены привлекательные расценки, а совершить покупку получится в любое время.
https://vote114.com/bbs/board.php?bo_table=free&wr_id=1136304
Visit https://qxinfo.online/ and learn about binary options at the next level. There are over a hundred different global trading assets available. You can also download the app for instant access to trading anywhere. More details on the website.
Фрибеты в букмекерской конторе Winline – отличная возможность получить бесплатные ставки на спортивные события. Чтобы получить фрибет, обычно необходимо выполнить определенные условия, например, зарегистрироваться на сайте, сделать первый депозит или разместить определенную ставку. Как только условия выполнены, фрибет будет доступен для активации. Список всех фрибетов Винлайн – https://t.me/s/freebet_winline
Вам срочно нужны денежные средства? Мы готовы оказать оперативную и своевременную помощь в трудную минуту, расскажем вам, что представляет из себя онлайн займ и как получить его. Гарантируем гибкие условия и персональный подход к абсолютно каждому клиенту. Ищете кредит онлайн заявка без справок? Newloans.ru – сайт, которым очень удобно пользоваться. Здесь представлена только полезная информация. Теперь не надо просить у близких и знакомых взаймы, с нами вы реализуете свои задумки. Просто оформите заявку, дождитесь решения и получите деньги.
Хотите смотреть в великолепном качестве онлайн русские фильмы и сериалы? Портал russkie-serial.net предоставляет такую прекрасную возможность. Здесь вы найдете детектив, фантастику, мелодраму, мистику, криминал, комедию, боевик, триллер и многое другое. Ищете русские мелодрамы новинки смотреть онлайн? Russkie-Serial.net – сайт, команда которого приготовила лучшие фильмы, смотрите их уже сегодня. Также у вас есть возможность оставить о кино свой комментарий. Для этого необходимо указать свой e-mail и имя, написать текст и нажать на «Отправить». Уверены в том, что вам понравится наш портал.
Зайдите на сайт https://aerosys.ru/ и вы сможете ознакомиться с услугами компании Авиа Спринт Карго – такими как грузовые авиаперевозки и полный спектр связанных с ними услуг. Вы сможете рассчитать стоимость доставки и отслеживать груз онлайн по номеру накладной. Подробнее обо всех услугах на сайте.
является участником банды . Именно помогла ему открыть сайт и теперь она за своё покровительство и раскрутку получает часть денег от клиентов-лохов, которых обработал мерзавец-шарлатан.
Как это обычно и бывает у мошенников, — это не настоящие имя-отчество, а очередной псевдоним. За случайной кличкой скрывается безработный, раньше уже мотавший сроки, продолжающий свою мошенническую деятельность.
Вместо реального фото, на вывешено сгенерированное нейросетью изображение мультяшного персонажа, эдакого старца-волхва. Настоящую же свою испитую,физиономию немаг тщательно скрывает, так как справедливо опасается, что вычислят и накажут.
Также на размещены написанные аферистом отзывы даже за 2010 год. В реальности же сайт появился всего пару недель назад – 15 февраля 2024 года. И именно эти две недели уголовник обманывает именно под кличкой.
Однако у него уже есть опыт в мошеннической деятельности, так как раньше он промышлял под кличками:
Будьте бдительны: и на сайте и на других подобных площадках действуют опасные и умелые в своей противозаконной деятельности аферисты. Они знают, как войти в доверие, но никто из них не способен оказать реальную помощь.
И шарлатан и подобные ему, постоянно дают пустые обещания. Деньги гребут лопатой, но никогда ни одному человеку не помогут. Так как все приписываемые им сверхъестественные способности — один сплошной обман. Ни один реальный отзыв от обманутых клиентов на не будет пропущен лжемагом – все отзывы от сам себе и пишет.
шарлатан аниме
На сайте https://svetodar.pro/ запишитесь онлайн в офтальмологическую клинику для всей семьи «Светодар», где работают настоящие, квалифицированные и компетентные специалисты. Они помогут вернуть зрение и радость жизни. Имеется не только взрослое, но и детское отделение, где всегда найдут правильный подход к ребенку и помогут улучшить зрение. Врачи объяснят диагноз, а также назначат схему лечения. Более чем 13 лет врачи заботятся о зрении своих пациентов. В клиниках работают более 50 лучших врачей.
Доброго!
Как гарант сервис помогает музыкантам защитить свои авторские права. Гарант сервис обеспечивает музыкантам надежную защиту и уверенность в законности и легальности их творчества.
Благодаря этому сервису музыканты могут быть уверены в том, что их каверы будут защищены от незаконного использования и доступны для публичного прослушивания и монетизации.
Таким образом, гарант сервис помогает музыкантам сфокусироваться на творческом процессе, не беспокоясь о возможных юридических проблемах.
https://known-brands.ru/295976-kak-zarabotat-na-kaverakh-onlajjn-3n/
[url=https://publicists.ru/7937639-kak-sdelat-kaver-na-pesnyu-vgj/] Как получить разрешение на кавер песни? [/url]
Распространение и лицензирование кавер-версий
Лицензирование каверов
Монетизация кавер-версий
Через каверы в звезды! Доказано!
Как получить разрешение на кавер
Как сделать кавер на песню
Выпустить свою музыку на цифровых площадках
Кавер-версии в эстрадной вокальной музыке
Могу ли я выкладывать свои каверы на Apple Music?
Выложить кавер на все площадки
Удачи и хорошего вайба!
В поисках где [url=https://el-gusto.ru/]реплика наушников Airpods PRO[/url]? Топовые беспроводные наушники в Москве и РФ. Реплика оригинальных AirPods с шумоподавлением по скидке. Только проверенные гарнитуры по низким ценам. Быстро доставим по России.
Ищете где [url=https://el-gusto.ru/]копия наушников Airpods PRO[/url]? Наилучшие беспроводные наушники в Москве и области. Реплика оригинальных AirPods с активным шумоподавлением со скидкой. Только проверенные гаджеты по доступным ценам. Быстрая доставка по России.
Искали где [url=https://el-gusto.ru/]el-gusto.ru[/url]? Качественные беспроводные наушники в Москве и области. Копия оригинальных AirPods с шумоподавлением всего за 2490 рублей. Самые качественные гаджеты по доступным ценам. Доставка по России.
Ищете где [url=https://el-gusto.ru/]купить копию Аирподс ПРО в Москве[/url]? Качественные беспроводные наушники в Москве и области. Копия оригинальных AirPods с активным шумоподавлением со скидкой. Самые надежные гарнитуры по приемлимым ценам. Доставим по России.
Ищете где [url=https://el-gusto.ru/]купить копию наушников Airpods PRO[/url]? Лучшие беспроводные наушники в Москве. Копия оригинальных AirPods с шумоподавлением по скидке. Надежные гаджеты по приемлимым ценам. Быстрая доставка по России.
Зайдите на сайт https://used-cars.ru/ и вы сможете купить в Москве автомобиль с пробегом (б/у) от БорисХоф. Продажа подержанных автомобилей, все машины проверены и находятся в Москве. Большой каталог БУ автомобилей с пробегом от официального дилера БорисХоф, узнайте на сайте цены и характеристики на бу авто.
Ищете где [url=https://el-gusto.ru/]копия Airpods PRO[/url]? Лучшие беспроводные наушники в Москве. Реплика оригинальных AirPods с шумоподавлением по цене 2490 рублей. Самые качественные гарнитуры по приемлимым ценам. Доставим по России.
Искали где [url=https://el-gusto.ru/]купить копию Аирподс ПРО[/url]? Топовые беспроводные наушники в Москве и области. Реплика оригинальных AirPods с шумоподавлением по скидке. Только качественные гаджеты по доступным ценам. Доставка по России.
Искали где [url=https://el-gusto.ru/]реплика Airpods PRO[/url]? Топовые беспроводные наушники в Москве и РФ. Реплика оригинальных AirPods с шумоподавлением по скидке. Проверенные гарнитуры по приемлимым ценам. Доставка по России.
Искали где [url=https://el-gusto.ru/]купить реплику Аирподс ПРО[/url]? Качественные беспроводные наушники в Москве и области. Копия оригинальных AirPods с активным шумоподавлением со скидкой. Только проверенные гарнитуры по низким ценам. Доставка по России.
Зачем рекламировать этот левый обзорник casinozeus.by одни мошенники. [url=https://rsute.ru/1005162-igornyj-biznes-v-belarusi-v-2021-godu-mnenie-eksperta-casino-zeus.html]http://forum.igromania.ru/member.php?u=630810[/url]
На сайте https://otzovik-rasteniya.ru/ вы можете почитать и оставить честные отзывы о питомниках, а также растениях. Добавьте отзыв прямо сейчас, если вы уже воспользовались услугами компании. Так вы узнаете о том, какие виды растений больше всего подходят для посадки на вашей даче с учетом особенностей ее расположения. Покупатели смогут быстро сориентироваться в изобилии магазинов, в которых получится приобрести любые виды растений. Вы узнаете, какие из них подходят для выращивания дома.
Будто других нормальных сайтов нет зациклились на casinozeus как ненормальные. [url=https://www.vostlit.info/misc/populyarnyj-ekspert-sajta-casino-zeus-rasskazal-pro-luchshie-onlajn-kazino-v-belarusi.html]https://www.presa.com.ua/rozvahy/vse-ob-igrovykh-avtomatakh-v-belorussii-istoriya-sozdaniya-printsipy-igry-i-gde-najti-podkhodyashchee-kazino-dlya-igry-v-sloty-rejting-alekseya-ivanova.html[/url]
Ссылки на [url=https://rsute.ru/1005162-igornyj-biznes-v-belarusi-v-2021-godu-mnenie-eksperta-casino-zeus.html]сборник казиношек от зевса[/url] разве что для профитов общий развод.
https://game24.space/
Раз за разом замечаю [url=https://www.vostlit.info/misc/populyarnyj-ekspert-sajta-casino-zeus-rasskazal-pro-luchshie-onlajn-kazino-v-belarusi.html]казино-лохотроны[/url] в рекомендациях сборника зевс.
Чуваки ни в коем случае не покупайтесь на [url=https://actualtraffic.ru/post/post-744002/]сборник казиношек от зевса развод[/url] полнейший.
Бросьте вы эту пародию на обзорник казино зевс не позорьтесь уже достали. [url=http://forum.igromania.ru/member.php?u=630810]https://actualtraffic.ru/post/post-744002/[/url]
Чем дальше от [url=http://m.fgis.economy.minregion.ru/publication/5296-aleksey-ivanov-rasskazal-o-populyarnyh-bonusah-v-onlayn-kazino-belarusi.html]casinozeus.by[/url] тем лучше сплошная реклама мутных контор.
Вечно виснет и не грузится этот обзорник зевс техническое отставание. [url=https://obyava.ua/ru/blog/aleksey-ivanov-o-bezdepozitnyh-bonusah-dlya-igrokov-v-kazino-onlayn.html]https://www.globalmsk.ru/firmnews/id/33207/1[/url]
Какими-то студентами писались отзывы для [url=https://obyava.ua/ru/blog/aleksey-ivanov-o-bezdepozitnyh-bonusah-dlya-igrokov-v-kazino-onlayn.html]детища александра иванова[/url].
https://podacha-blud.com/
Изучение финансов и инвестиций становится всё более важным в современном мире, особенно с развитием доступности криптовалют и трейдинга. Многим людям недостает знаний в этой области. Поэтому, сайт https://forexoptimum.ru является незаменимым ресурсом для тех, кто стремится развиваться финансово и получать доход. Тут найдете множество полезной информации об аспектах финансового мира, включая акции, облигации, криптовалюты и другие виды инвестиций
Ищете где [url=https://mdou41orel.ru/]купить копию наушников Airpods PRO[/url]? Топовые беспроводные наушники в Москве и РФ. Копия оригинальных AirPods с активным шумоподавлением по скидке. Проверенные гаджеты по низким ценам. Быстрая доставка по России.
Искали где [url=https://mdou41orel.ru/]копия Airpods PRO[/url]? Наилучшие беспроводные наушники в Москве и области. Реплика оригинальных AirPods с активным шумоподавлением по скидке. Самые надежные гарнитуры по низким ценам. Доставка по России.
Искали где [url=https://mdou41orel.ru/]купить реплику наушников Airpods PRO[/url]? Топовые беспроводные наушники в Москве. Реплика оригинальных AirPods с шумоподавлением всего за 2490 рублей. Надежные гаджеты по доступным ценам. Быстро доставим по России.
Искали где [url=https://mdou41orel.ru/]реплика Airpods PRO[/url]? Топовые беспроводные наушники в Москве и РФ. Копия оригинальных AirPods с активным шумоподавлением по скидке. Надежные гаджеты по низким ценам. Быстро доставим по России.
Ищете где [url=https://mdou41orel.ru/]купить реплику Airpods PRO[/url]? Качественные беспроводные наушники в Москве. Копия оригинальных AirPods с шумоподавлением со скидкой. Надежные гарнитуры по приемлимым ценам. Быстрая доставка по России.
Искали где [url=https://mdou41orel.ru/]купить реплику Аирподс ПРО[/url]? Лучшие беспроводные наушники в Москве. Копия оригинальных AirPods с активным шумоподавлением со скидкой. Надежные гарнитуры по приемлимым ценам. Доставим по России.
В поисках где [url=https://mdou41orel.ru/]копия Airpods PRO[/url]? Топовые беспроводные наушники в Москве и области. Реплика оригинальных AirPods с шумоподавлением со скидкой. Надежные гаджеты по доступным ценам. Быстрая доставка по России.
https://gruzchikirabotnik.ru/
Ищете где [url=https://mdou41orel.ru/]купить копию Аирподс ПРО в Москве[/url]? Лучшие беспроводные наушники в Москве и области. Реплика оригинальных AirPods с активным шумоподавлением по скидке. Проверенные гаджеты по приемлимым ценам. Быстро доставим по России.
Искали где [url=https://mdou41orel.ru/]купить реплику Аирподс ПРО[/url]? Качественные беспроводные наушники в Москве. Реплика оригинальных AirPods с шумоподавлением со скидкой. Только качественные гаджеты по приемлимым ценам. Доставим по России.
Ищете где [url=https://mdou41orel.ru/]купить копию Аирподс ПРО в Москве[/url]? Наилучшие беспроводные наушники в Москве. Реплика оригинальных AirPods с шумоподавлением по цене 2490 рублей. Только качественные гаджеты по доступным ценам. Доставка по России.
Ищете где [url=https://mdou41orel.ru/]mdou41orel.ru[/url]? Лучшие беспроводные наушники в Москве и РФ. Копия оригинальных AirPods с активным шумоподавлением со скидкой. Самые надежные гаджеты по приемлимым ценам. Быстро доставим по России.
Ищете где [url=https://mdou41orel.ru/]купить реплику Аирподс ПРО в Москве[/url]? Качественные беспроводные наушники в Москве и области. Реплика оригинальных AirPods с активным шумоподавлением со скидкой. Надежные гарнитуры по приемлимым ценам. Быстрая доставка по России.
ТопКлиматДВ – интернет-магазин, специализирующийся на климатическом оборудовании. Поставки выполняем в любой регион России. Предлагаем высокое качество обслуживания, доступные цены и предоставляем длительную гарантию на все позиции. Ищете где купить инверторный кондиционер? Топклиматдв.рф – сайт, где найдете то, что нужно для создания комфортной атмосферы в вашем доме. Вы можете самостоятельно выбрать удобный способ доставки и оплаты. Если хотите сделать заказ на новейшую модель климатического оборудования с необходимостью монтажа «под ключ», то вы попали точно по адресу!
Искали где [url=https://mdou41orel.ru/]купить копию наушников Airpods PRO[/url]? Качественные беспроводные наушники в Москве. Реплика оригинальных AirPods с шумоподавлением по цене 2490 рублей. Только качественные гаджеты по низким ценам. Быстрая доставка по России.
Ищете где [url=https://mdou41orel.ru/]mdou41orel.ru[/url]? Наилучшие беспроводные наушники в Москве. Копия оригинальных AirPods с активным шумоподавлением по цене 2490 рублей. Проверенные гаджеты по низким ценам. Быстрая доставка по России.
Ищете где [url=https://mdou41orel.ru/]копия Airpods PRO[/url]? Лучшие беспроводные наушники в Москве и РФ. Реплика оригинальных AirPods с шумоподавлением по скидке. Проверенные гарнитуры по доступным ценам. Быстрая доставка по России.
Ищете где [url=https://mdou41orel.ru/]реплика Airpods PRO[/url]? Топовые беспроводные наушники в Москве и РФ. Реплика оригинальных AirPods с шумоподавлением всего за 2490 рублей. Только качественные гаджеты по доступным ценам. Быстро доставим по России.
Internet Store гарантирует доступную стоимость и предлагает услугу «аренда сайта». Наши высококвалифицированные специалисты понимают, как все должно работать. Они помогут развить новый бизнес или же расширить действующий. Ищете аренда сайта Internet-Store.online – сайт, где можете ознакомиться с условиями аренды. Здесь мы развернуто ответили на самые популярные вопросы. Если желаете получить консультацию, позвоните нам по телефону. Мы точно знаем, как привлечь клиентов и запустить действенные инструменты продвижения!
В поисках где [url=https://mdou41orel.ru/]купить копию Airpods PRO в Москве[/url]? Наилучшие беспроводные наушники в Москве. Реплика оригинальных AirPods с активным шумоподавлением со скидкой. Проверенные гаджеты по приемлимым ценам. Быстро доставим по России.
Ищете где [url=https://mdou41orel.ru/]реплика Airpods PRO[/url]? Наилучшие беспроводные наушники в Москве и РФ. Реплика оригинальных AirPods с активным шумоподавлением со скидкой. Только проверенные гаджеты по приемлимым ценам. Быстро доставим по России.
https://onff.ru/sistema-kontrolya-rabochego-vremeni-sprutmonitor/
https://muslim-prayer-times.com/
Здравствуйте!
White Queen продолжает радовать своих фанатов новыми хитами. Трек БАС – это взрывная смесь энергии и страсти, которая создает неповторимую атмосферу на танцполах по всему миру. Слушайте и наслаждайтесь этим гимном рейверов!
White Queen радует поклонников новым танцевальным треком в стиле рейв! Певица White Queen порадовала своих поклонников новым треком под названием “БАС”. Эта энергичная композиция в стиле рейв уже завоевала сердца слушателей и вызвала восторг у фанатов. White Queen продолжает удивлять своим творчеством и не перестает удивлять своих поклонников новыми хитами.
White Queen представила свою новую песню “БАС” – аудитория в восторге! После долгого ожидания певица White Queen выпустила свой новый трек “БАС”. Эта танцевальная композиция в стиле рейв сразу же стала хитом и завоевала популярность среди слушателей. Многие считают, что эта песня – настоящее открытие для певицы и признают, что White Queen превзошла саму себя.
https://music.yandex.ru/track/123467211
[url=https://music.youtube.com/watch?v=PdQ9WEDH-Pg] послушать музыку [/url]
песня про рейв
слушать музыку юрий антонов
слушать музыку зима в сердце
послушать армянскую музыку
сайт слушать музыку
рейв слушать онлайн бесплатно
слушать музыку онлайн 2023
где послушать живую музыку в екатеринбурге
слушать музыку мираж
как в вк послушать музыку друзей
Удачи и хорошего вайба!
На сайте https://izhevsk.soho.menu/ вы сможете заказать вкусные и аппетитные сеты, экзотические роллы, ароматную и свежую пиццу, сытные суши, разнообразные закуски, интересные десерты, созданные из отборных ингредиентов. Также здесь можно заказать и свежайший шашлык из нежного мяса. Рекомендуется попробовать сеты, созданные из самых разнообразных ингредиентов. Они порадуют удивительным калейдоскопом вкусов. Каждый день выкладываются удивительные и интересные предложения, которые вы сможете приобрести с хорошей скидкой. Действуют ограниченное количество времени.
Ищете вадим зеланд? Посетите сайт Zelands.ru и Вы узнаете кто такой кто такой Вадим Зеланд и что такое трансерфинг. Трансерфинг зеланда уникальная технология достижения целей которая позволит вам управлять событиями, а зеланд является лучшим специалистом в этой области. Трансерфинг реальности Вадим Зеланд зайдите на сайт и узнайте больше.
На сайте https://xn--90abjmfe8alfadz.xn--p1ai/ закажите срубы домов, бань в СПб, Ленинградской области. В результате вы получите качественный и практичный дом, который наделен длительными сроками эксплуатации. Этот материал считается экологически чистым и безопасным, а потому не окажет негативного влияния на здоровье. Все строения возводятся лучшими, высококлассными специалистами, у которых огромный опыт за плечами, а также талант. Все дома отличаются привлекательным и интересным дизайном.
Одни проблемы. [url=https://leon.ru/android/]Программа бк леон не устанавливается[/url] и с чем связана данная проблема я не знаю. Но лучше поискать другой источник для скачивания данной программы. Тут тратить свое время не советую.
На сайте https://tradershero.com/ вы легко сможете найти инвестиционные инструменты для реализации ваших финансовых потребностей. У нас на сайте есть возможность получать бонусы и выплаты, для этого стоит зарегистрироваться, выбрать компанию и открыть реальный торговый счет. Здесь вы найдете самые лучшие криптовалютные предложения, а также экспертные обзоры торговых компаний. Помимо прочего, на нашем портале можно ознакомиться с отзывами пользователей. С нашей помощью вы выберите лучшего брокера.
Одни проблемы. [url=https://leon.ru/android/]Букмекерская контора леон не работает[/url] и с чем связана данная проблема я не знаю. Но лучше поискать другой источник для скачивания данной программы. Тут тратить свое время не советую.
Одни проблемы. [url=https://leon.ru/android/]Почему не работает бк леон сегодня[/url] и с чем связана данная проблема я не знаю. Но лучше поискать другой источник для скачивания данной программы. Тут тратить свое время не советую.
Зайдите на сайт https://top-setka.ru/catalog/setka_rabitsa/ и вы сможете купить сетка рабица недорого в Москве и МО. Акция бесплатный расчет! Сетка рабица металлическая, сетка рабица оцинкованная всегда в наличии по выгодной цене от производителя. Подробнее на сайте.
Одни проблемы. [url=https://leon.ru/android/]Приложение леон ошибка[/url] и с чем связана данная проблема я не знаю. Но лучше поискать другой источник для скачивания данной программы. Тут тратить свое время не советую.
Одни проблемы. [url=https://leon.ru/android/]Почему не работает бк леон сегодня[/url] и с чем связана данная проблема я не знаю. Но лучше поискать другой источник для скачивания данной программы. Тут тратить свое время не советую.
Одни проблемы. [url=https://leon.ru/android/]Леон букмекерская не работает[/url] и с чем связана данная проблема я не знаю. Но лучше поискать другой источник для скачивания данной программы. Тут тратить свое время не советую.
Одни проблемы. [url=https://leon.ru/android/]Почему не работает леон букмекерская контора[/url] и с чем связана данная проблема я не знаю. Но лучше поискать другой источник для скачивания данной программы. Тут тратить свое время не советую.
Одни проблемы. [url=https://leon.ru/android/]Леон не работает приложение[/url] и с чем связана данная проблема я не знаю. Но лучше поискать другой источник для скачивания данной программы. Тут тратить свое время не советую.
Одни проблемы. [url=https://leon.ru/android/]Почему не работает приложение леон[/url] и с чем связана данная проблема я не знаю. Но лучше поискать другой источник для скачивания данной программы. Тут тратить свое время не советую.
Одни проблемы. [url=https://apps.rustore.ru/app/com.leonru.mobile5]Почему не работает бк леон сегодня[/url] и с чем связана данная проблема я не знаю. Но лучше поискать другой источник для скачивания данной программы. Тут тратить свое время не советую.
Одни проблемы. [url=https://apps.rustore.ru/app/com.leonru.mobile5]Почему не работает бк леон[/url] и с чем связана данная проблема я не знаю. Но лучше поискать другой источник для скачивания данной программы. Тут тратить свое время не советую.
Одни проблемы. [url=https://apps.rustore.ru/app/com.leonru.mobile5]Почему не работает приложение леон[/url] и с чем связана данная проблема я не знаю. Но лучше поискать другой источник для скачивания данной программы. Тут тратить свое время не советую.
Одни проблемы. [url=https://apps.rustore.ru/app/com.leonru.mobile5]Почему не работает леон букмекерская контора[/url] и с чем связана данная проблема я не знаю. Но лучше поискать другой источник для скачивания данной программы. Тут тратить свое время не советую.
Одни проблемы. [url=https://apps.rustore.ru/app/com.leonru.mobile5]Леон не работает приложение[/url] и с чем связана данная проблема я не знаю. Но лучше поискать другой источник для скачивания данной программы. Тут тратить свое время не советую.
Одни проблемы. [url=https://apps.rustore.ru/app/com.leonru.mobile5]БК леон не работает[/url] и с чем связана данная проблема я не знаю. Но лучше поискать другой источник для скачивания данной программы. Тут тратить свое время не советую.
В поисках где [url=https://el-gusto.ru/]купить реплику Аирподс ПРО[/url]? Топовые беспроводные наушники в Москве. Копия оригинальных AirPods с шумоподавлением со скидкой. Проверенные гарнитуры по низким ценам. Доставка по России.
Искали где [url=https://el-gusto.ru/]купить копию наушников Airpods PRO[/url]? Наилучшие беспроводные наушники в Москве и РФ. Реплика оригинальных AirPods с шумоподавлением со скидкой. Проверенные гаджеты по низким ценам. Доставка по России.
В поисках где [url=https://el-gusto.ru/]купить реплику наушников Airpods PRO[/url]? Качественные беспроводные наушники в Москве. Копия оригинальных AirPods с активным шумоподавлением по скидке. Только проверенные гарнитуры по низким ценам. Доставим по России.
В поисках где [url=https://el-gusto.ru/]реплика наушников Airpods PRO[/url]? Лучшие беспроводные наушники в Москве. Реплика оригинальных AirPods с активным шумоподавлением всего за 2490 рублей. Самые качественные гарнитуры по низким ценам. Быстрая доставка по России.
Ищете где [url=https://el-gusto.ru/]купить реплику наушников Airpods PRO[/url]? Качественные беспроводные наушники в Москве и РФ. Реплика оригинальных AirPods с шумоподавлением по цене 2490 рублей. Самые качественные гаджеты по приемлимым ценам. Быстро доставим по России.
В поисках где [url=https://el-gusto.ru/]купить реплику наушников Airpods PRO[/url]? Наилучшие беспроводные наушники в Москве и области. Копия оригинальных AirPods с активным шумоподавлением по скидке. Надежные гарнитуры по приемлимым ценам. Быстро доставим по России.
Искали где [url=https://el-gusto.ru/]купить копию Airpods PRO в Москве[/url]? Лучшие беспроводные наушники в Москве и РФ. Копия оригинальных AirPods с шумоподавлением со скидкой. Только качественные гаджеты по низким ценам. Доставим по России.
Искали где [url=https://el-gusto.ru/]https://el-gusto.ru/[/url]? Качественные беспроводные наушники в Москве и области. Копия оригинальных AirPods с активным шумоподавлением со скидкой. Самые качественные гарнитуры по приемлимым ценам. Быстро доставим по России.
Ищете где [url=https://el-gusto.ru/]купить копию Airpods PRO[/url]? Топовые беспроводные наушники в Москве и РФ. Реплика оригинальных AirPods с шумоподавлением всего за 2490 рублей. Самые надежные гарнитуры по низким ценам. Доставка по России.
Visit the website download spaceman predictor – to learn about the advantages of Predictor spaceman. Spaceman Predictor – increase your chances of winning in the exciting world of predictor spaceman version 2.0. You can download spaceman predictor online now. The entire review of the predictor version 2.0 spaceman application can be found on the website.
Тайсинь – компания, которая оказывает услуги по подбору и поиску нужного оборудования. Предлагаем станки из Китая с быстрой доставкой оптом. Не нашли подходящие? Свяжитесь с нами, мы проконсультируем и подберем то, что вам действительно нужно. Ищете технологические линии китайского производства? Taixinasia.com – сайт, где у вас есть возможность найти качественное китайское оборудование, которое неплохо справляется с задачами. Для получения подробной информации по стоимости доставки оборудования, обратитесь к грамотным специалистам нашей компании. Рады вам помочь!
Искали где [url=https://mdou41orel.ru/]купить реплику Аирподс ПРО в Москве[/url]? Топовые беспроводные наушники в Москве и РФ. Реплика оригинальных AirPods с активным шумоподавлением по цене 2490 рублей. Только проверенные гарнитуры по низким ценам. Быстро доставим по России.
услуги грузчиков недорого
Искали где [url=https://mdou41orel.ru/]купить реплику Аирподс ПРО в Москве[/url]? Лучшие беспроводные наушники в Москве и РФ. Копия оригинальных AirPods с шумоподавлением по скидке. Самые качественные гарнитуры по низким ценам. Быстро доставим по России.
Ищете где [url=https://mdou41orel.ru/]купить копию Аирподс ПРО в Москве[/url]? Лучшие беспроводные наушники в Москве и РФ. Реплика оригинальных AirPods с шумоподавлением по скидке. Надежные гаджеты по доступным ценам. Доставим по России.
Доброго!
От местных инициатив до городских праздников: top10.kr.ua охватывает все стороны жизни Кропивницкого.
Ждем Вас на https://top10.kr.ua/category/news-ukraine/
[url=https://top10.kr.ua/category/news-ukraine/]Новости Украины сейчас[/url]
главные новости Кропивницкого
главные новости Украины
новости Кропивницкого ДТП
Удачи и хороших новостей!
Искали где [url=https://mdou41orel.ru/]купить копию наушников Airpods PRO[/url]? Качественные беспроводные наушники в Москве. Копия оригинальных AirPods с активным шумоподавлением со скидкой. Проверенные гарнитуры по доступным ценам. Доставим по России.
Ищете где [url=https://mdou41orel.ru/]купить реплику наушников Airpods PRO в Москве[/url]? Наилучшие беспроводные наушники в Москве и РФ. Копия оригинальных AirPods с активным шумоподавлением со скидкой. Надежные гаджеты по низким ценам. Быстро доставим по России.
На сайте http://hyggehouse23.su забронируйте номер в «HYGGE-HOUSE». Вам точно понравится удивительный, завораживающий отдых на природе, среди живописных видов. Вас поразит комфорт, а также роскошь, которая царит в доме. Глемпинг находится в горах и окружении леса. Вы сможете устроить незабываемый отдых вместе с домашним животным. На территории имеется баня, где вы сможете отдохнуть в специальной комнате, а затем искупаться в бассейне. В нем чистая, приятная вода. Также к вашим услугам и прокат Эндуро. Имеются варианты различных размеров, моделей.
В поисках где [url=https://mdou41orel.ru/]купить копию Аирподс ПРО в Москве[/url]? Лучшие беспроводные наушники в Москве и РФ. Копия оригинальных AirPods с шумоподавлением всего за 2490 рублей. Проверенные гаджеты по доступным ценам. Доставка по России.
В поисках где [url=https://mdou41orel.ru/]купить копию Airpods PRO в Москве[/url]? Лучшие беспроводные наушники в Москве. Реплика оригинальных AirPods с активным шумоподавлением со скидкой. Надежные гарнитуры по доступным ценам. Доставим по России.
В поисках где [url=https://mdou41orel.ru/]mdou41orel.ru[/url]? Лучшие беспроводные наушники в Москве и области. Копия оригинальных AirPods с активным шумоподавлением по скидке. Только качественные гарнитуры по доступным ценам. Быстро доставим по России.
В поисках где [url=https://mdou41orel.ru/]mdou41orel.ru[/url]? Топовые беспроводные наушники в Москве. Копия оригинальных AirPods с шумоподавлением всего за 2490 рублей. Только качественные гаджеты по доступным ценам. Доставим по России.
В поисках где [url=https://el-gusto.ru/]купить копию наушников Airpods PRO[/url]? Качественные беспроводные наушники в Москве. Реплика оригинальных AirPods с активным шумоподавлением по скидке. Надежные гарнитуры по приемлимым ценам. Доставим по России.
Искали где [url=https://el-gusto.ru/]купить реплику Airpods PRO в Москве[/url]? Наилучшие беспроводные наушники в Москве. Копия оригинальных AirPods с шумоподавлением всего за 2490 рублей. Только качественные гаджеты по низким ценам. Доставим по России.
Ищете где [url=https://el-gusto.ru/]el-gusto.ru[/url]? Качественные беспроводные наушники в Москве. Копия оригинальных AirPods с шумоподавлением по цене 2490 рублей. Самые надежные гаджеты по низким ценам. Доставим по России.
Pingback: buspar drug
Искали где [url=https://el-gusto.ru/]el-gusto.ru[/url]? Топовые беспроводные наушники в Москве и РФ. Копия оригинальных AirPods с шумоподавлением по цене 2490 рублей. Только качественные гаджеты по приемлимым ценам. Быстро доставим по России.
https://potreb-prava.com/
В поисках где [url=https://el-gusto.ru/]купить реплику наушников Airpods PRO в Москве[/url]? Лучшие беспроводные наушники в Москве и области. Копия оригинальных AirPods с активным шумоподавлением по скидке. Только проверенные гаджеты по низким ценам. Доставка по России.
На сайте https://ecodok.ru/ вы сможете воспользоваться такими услугами, как: разработка экологической документации, экологическое сопровождение, подготовка к проверке соответствующими органами, экологический контроль. Прямо сейчас получите бесплатную консультацию. Просто заполните форму, чтобы консультанты компании связались с вами в ближайшее время. Все услуги предоставляются в минимальные сроки, по доступной стоимости. В компании работают первоклассные специалисты, которые трудятся на профессиональном уровне.
В поисках где [url=https://el-gusto.ru/]купить реплику Airpods PRO в Москве[/url]? Наилучшие беспроводные наушники в Москве. Реплика оригинальных AirPods с шумоподавлением всего за 2490 рублей. Надежные гарнитуры по низким ценам. Доставка по России.
Искали где [url=https://el-gusto.ru/]купить копию наушников Airpods PRO[/url]? Наилучшие беспроводные наушники в Москве. Реплика оригинальных AirPods с активным шумоподавлением со скидкой. Самые качественные гаджеты по доступным ценам. Доставим по России.
Ищете где [url=https://el-gusto.ru/]https://el-gusto.ru/[/url]? Лучшие беспроводные наушники в Москве и области. Копия оригинальных AirPods с шумоподавлением со скидкой. Только качественные гаджеты по приемлимым ценам. Доставим по России.
Привет любители софта!
Хотите скачивать торренты максимально просто и эффективно?
Тогда uFiler – идеальное решение для вас. Это бесплатная программа с продуманным интерфейсом, который позволяет легко управлять загрузками.
Вы можете добавлять неограниченное количество торрентов, настраивать скорость скачивания, а также отслеживать прогресс загрузок в режиме реального времени.
Функциональность uFiler впечатляет, а бесплатное распространение делает его доступным для всех.
Загрузите приложение по ссылке https://ufile-for-pc.ru/ и оцените его преимущества!
[url=https://ufile-for-pc.ru/]ufiler что это за программа[/url]
ufiler скачать на пк бесплатно
ufiler что это за программа
скачать ufiler
ufiler скачать торрент
Удачи и хорошего серфинга по торрентам!
Ищете где [url=https://mdou41orel.ru/]купить лучшую реплику Airpods PRO[/url]? Топовые беспроводные наушники в Москве. Реплика оригинальных AirPods с активным шумоподавлением всего за 2490 рублей. Только проверенные гаджеты по низким ценам. Доставим по России.
дымоходы
Перед покупкой печи или камина обязательно проконсультируйтесь о возможности установки дымохода в вашем доме.
Искали где [url=https://mdou41orel.ru/]купить реплику Airpods PRO[/url]? Качественные беспроводные наушники в Москве. Реплика оригинальных AirPods с активным шумоподавлением по цене 2490 рублей. Самые качественные гаджеты по приемлимым ценам. Быстро доставим по России.
В поисках где [url=https://mdou41orel.ru/]копия наушников Airpods PRO[/url]? Наилучшие беспроводные наушники в Москве. Копия оригинальных AirPods с активным шумоподавлением со скидкой. Только проверенные гаджеты по низким ценам. Доставим по России.
Ищете где [url=https://mdou41orel.ru/]купить реплику Аирподс ПРО в Москве[/url]? Топовые беспроводные наушники в Москве и РФ. Копия оригинальных AirPods с шумоподавлением всего за 2490 рублей. Только проверенные гарнитуры по низким ценам. Быстрая доставка по России.
В поисках где [url=https://mdou41orel.ru/]купить копию Аирподс ПРО в Москве[/url]? Топовые беспроводные наушники в Москве и РФ. Копия оригинальных AirPods с шумоподавлением по скидке. Самые качественные гаджеты по низким ценам. Быстро доставим по России.
Ищете где [url=https://mdou41orel.ru/]купить реплику наушников Airpods PRO[/url]? Лучшие беспроводные наушники в Москве. Копия оригинальных AirPods с шумоподавлением по скидке. Только проверенные гаджеты по приемлимым ценам. Доставка по России.
Искали где [url=https://mdou41orel.ru/]копия Airpods PRO[/url]? Лучшие беспроводные наушники в Москве и области. Реплика оригинальных AirPods с шумоподавлением по скидке. Только проверенные гаджеты по приемлимым ценам. Доставим по России.
Pingback: baclofen 10 milligrams
Pingback: celexa
Pingback: ashwagandha benefits for women
Доброго!
От политики до культуры: всеобъемлющие новости Украины и мира для вас.
Заходите https://wian.top/category/avto
[url=https://wian.top/category/fashion]шоу-бизнес новости [/url]
последние новости мира
новости Украины видео
экономические новости
Удачи и хороших новостей!
На сайте https://heno.by/ получите консультацию по поводу покупки оборудования для химической, калийной, нефтяной, а также газовой промышленности. Специально для вас установлена привлекательная и разумная стоимость, практикуется оперативная доставка. А на всю продукцию распространяются гарантии. Работа происходит по всей территории СНГ. Все товары реализуются напрямую от надежных и проверенных поставщиков, отвечающих за качество. Компания присутствует на рынке более 5 лет, предлагает товары более 14 производителей.
Внимание, любители ставок! БК Олимп представляет с гордостью последнюю версию своего приложения для Android, которое обещает перевернуть ваш мир ставок на радость. Мы обновили приложение, чтобы предоставить вам несравненный впечатление от ставок на спорт. [url=https://top-olimp-apk.ru/]Приложение Олимп[/url] и откройте просмотр разнообразных спортивных событий, который стал проще благодаря улучшенному интерфейсу и оптимизированной производительности данных. Присоединяйтесь к сообществу БК Олимп и пользуйтесь возможностью делать ставки где угодно, будь то дома или в путешествии. Загрузите новую версию приложения без задержек и погрузитесь в мир ставок с БК Олимп, где выигрыши становятся реальностью!
Pingback: celecoxib 400 mg
Одни проблемы. [url=https://leon.ru/android/]Почему не работает леон букмекерская контора[/url] и с чем связана данная проблема я не знаю. Но лучше поискать другой источник для скачивания данной программы. Тут тратить свое время не советую.
Pingback: bupropion sex drive
https://o-okkultizme.com
Одни проблемы. [url=https://leon.ru/android/]Почему не работает леон букмекерская контора[/url] и с чем связана данная проблема я не знаю. Но лучше поискать другой источник для скачивания данной программы. Тут тратить свое время не советую.
Pingback: celebrex 200mg price
Одни проблемы. [url=https://leon.ru/android/]Почему не работает бк леон сегодня[/url] и с чем связана данная проблема я не знаю. Но лучше поискать другой источник для скачивания данной программы. Тут тратить свое время не советую.
https://catherineasquithgallery.com
Одни проблемы. [url=https://leon.ru/android/]Не работает приложение бк леон[/url] и с чем связана данная проблема я не знаю. Но лучше поискать другой источник для скачивания данной программы. Тут тратить свое время не советую.
Одни проблемы. [url=https://leon.ru/android/]Почему не работает леон букмекерская контора[/url] и с чем связана данная проблема я не знаю. Но лучше поискать другой источник для скачивания данной программы. Тут тратить свое время не советую.
Гибкий мрамор и мраморный шпон от производителя https://td-gk.ru/ – зайдите на сайт и ознакомьтесь со всеми преимуществами нашей продукции для внутренней отделки помещений. Долговечный материал будет радовать вас продолжительное время, а помощь в монтаже реализует ваши решения для достижения идеального результата. Подробнее на сайте.
Одни проблемы. [url=https://apps.rustore.ru/app/com.leonru.mobile5]Приложение бк леон вылетает[/url] и с чем связана данная проблема я не знаю. Но лучше поискать другой источник для скачивания данной программы. Тут тратить свое время не советую.
Одни проблемы. [url=https://apps.rustore.ru/app/com.leonru.mobile5]Приложение бк леон вылетает[/url] и с чем связана данная проблема я не знаю. Но лучше поискать другой источник для скачивания данной программы. Тут тратить свое время не советую.
Одни проблемы. [url=https://apps.rustore.ru/app/com.leonru.mobile5]Почему не работает бк леон сегодня[/url] и с чем связана данная проблема я не знаю. Но лучше поискать другой источник для скачивания данной программы. Тут тратить свое время не советую.
Одни проблемы. [url=https://apps.rustore.ru/app/com.leonru.mobile5]Леон букмекерская не работает[/url] и с чем связана данная проблема я не знаю. Но лучше поискать другой источник для скачивания данной программы. Тут тратить свое время не советую.
Одни проблемы. [url=https://leon.ru/android/]БК леон не могу войти[/url] и с чем связана данная проблема я не знаю. Но лучше поискать другой источник для скачивания данной программы. Тут тратить свое время не советую.
услуги грузчиков городу
InLIBRARY – известная электронная библиотека научных публикаций, обладающая широкими возможностями поиска. Для регистрации необходимо ввести свои личные данные. Создайте новый аккаунт и путешествуйте с нами. Ищете научная электронная библиотека регистрация? InLibrary.uz – сайт библиотеки, где есть возможность быть читателем, который знакомится с размещенными работами и автором. Здесь вы найдете много чего полезного. Журналов, опубликованных на ресурсе 49. Вы узнаете, какова цель научной монографии и что это такое, посмотрите информацию на сайте уже сегодня.
Pro Studio окажет вам помощь в воплощении амбициозных проектов в реальность. Работая с нами, получите идеальных партнеров в достижении ваших целей. Мы специализируемся на создании высококачественных сайтов для разных бизнесов. Знаем, как сделать лучше, всегда учитываем требования и пожелания каждого клиента. Требуется создание сайта на bitrix? Iv-SEO.ru – здесь у вас есть возможность ознакомиться с услугами и узнать всю информацию о нашем digital-агентстве. Работаем как в Москве, так и в других регионах России. Свяжитесь с нами, чтобы узнать детальнее об условиях сотрудничества.
На сайте https://stroy-postavka.su воспользуйтесь возможностью приобрести песок непосредственно с карьера и с оперативной доставкой не только по Москве, но и области. В ассортименте имеется еще и мытый, сеянный, карьерный. Узнайте стоимость продукции прямо сейчас, чтобы сделать заказ. В эту компанию обращаются еще и по той причине, что в ней есть свой парк техники, что позволяет осуществить доставку в ближайшее время. Большой ассортимент позволит заказать, в том числе, нерудные материалы и по привлекательной цене.
На сайте https://apps.apple.com/ru/app/vava-sports-da/id6477537714 сыграйте в интересную, любопытную игру, созданную для того, чтобы проверить ваш уровень знаний, смекалку. Вас обрадует четкая графика, нетривиальный сюжет. Суть игры заключается в том, чтобы разгадать сюжет. Перед вами будет большое количество самых разных и увлекательных сюжетов, включая животных, природу, пейзажи, спорт, здания и многое другое. Игра отличается понятным, простым интерфейсом, а потому точно понравится всем. А на заднем плане есть приятная и негромкая музыка.
На сайте https://filmy.lords.lat/ у вас есть возможность смотреть бесплатно сериалы и фильмы в хорошем качестве. Здесь каждый подберет что-то интересное для себя, будь-то триллер, боевик, мелодрама или же детский мультик для ребенка. И все это удовольствие можно смотреть без рекламы и в любое время суток. Качественное кино даст возможность любому пользователю провести свое свободное время с пользой и весело. В каждой категории есть из чего выбрать, а это значит, что можно смотреть что-либо свежее.
Gogo Casino erbjuder ett brett utbud av spel och underhållning för sina spelare. För att säkerställa en bra spelupplevelse är det viktigt att ha tillgång till en pålitlig och effektiv kundsupport.
Gogo Casino har investerat i en dedikerad kundsupportavdelning för att kunna hjälpa sina spelare med eventuella frågor eller problem som kan uppstå under deras spelupplevelse. Kundsupportteamet är tillgängligt dygnet runt via e-post och livechatt för att ge spelarna snabb och professionell hjälp.
Genom att erbjuda en högkvalitativ kundsupport strävar Gogo Casino efter att skapa en trygg och säker miljö för sina spelare. Spelarna kan känna sig trygga när de vet att de kan få den hjälp de behöver när de behöver det.
Det är viktigt att ha tillgång till en pålitlig kundsupport när man spelar online, och Gogo Casino har tagit detta på stort allvar. Genom att investera i en dedikerad kundsupportavdelning visar Gogo Casino sitt engagemang för att ge spelarna den bästa möjliga spelupplevelsen.
Sammanfattningsvis är Gogo Casino kundsupport tillgänglig dygnet runt för att hjälpa spelarna med eventuella frågor eller problem som kan uppstå under deras spelupplevelse. Genom att erbjuda en högkvalitativ kundsupport strävar Gogo Casino efter att skapa en trygg och säker miljö för sina spelare.
https://gogocasino.one
Доброго!
Кропивницкий без прикрас: реальные новости и события, которые волнуют горожан.
Заходите https://0522.kr.ua/category/news-ukraine/
[url=https://0522.kr.ua/category/news-ukraine/]Новости Украины сегодня[/url]
главные новости Украины
новости Кропивницкого аварии
Удачи и хороших новостей!
Интернет магазин ножей Kusnica https://kusnica.ru/ – официальный сайт. Зайдите на сайт и ознакомьтесь с широким ассортиментом ножей российских и мировых брендов. Более 10000 товаров на сайте, а доставка осуществляется в любой регион. Все наши изделия сертифицированы. Также есть услуга ручной заточки ножей на профессиональном оборудовании. Подробнее на сайте.
какими криптовалютами можно расплачиваться Для каких целей криптовалюта является необходимой в Российской Федерации?
На сайте https://agro-met.ru/ подберите карданные валы по определенным параметрам. Они станут оптимальным решением как для оборудования, так и сельскохозяйственной промышленности. Компания находится на рынке более 20 лет, а потому детально выучила потребности клиентов, чтобы предложить им безупречный уровень сервиса, огромный ассортимент оборудования. Активное сотрудничество с различными производителями со всего мира. Здесь вы всегда найдете такое предложение, которое вам точно подойдет.
Зайдите на сайт Финансовой компании Бинкор https://binkor.ru/ – где вы сможете ознакомиться с различными программами кредитования частных лиц и бизнеса. Воспользуйтесь популярными предложениями или кредитным калькулятором для расчета стоимости кредита или проверьте кредитную историю. Подробнее на сайте.
Предлагаем слуги: снос дома, демонтаж фундамента, слом домов.
адрес вскрытие замков [url=http://www.azs-zamok13.ru/]http://www.azs-zamok13.ru/[/url] .
На сайте https://tecamet.ru/ вы можете приобрести прессы по приемлемой стоимости. Специалисты компании подберут для вас нужное оборудование, а если будет необходимо, произведут модификацию пресса, либо оборудования, которое соответствует вашему запросу. Прессы изготавливаются по чертежу клиента с нужными параметрами. Работаем со всеми регионами России, а также ближнего зарубежья. Приобретая продукцию у нас, вы можете не сомневаться в качестве и длительном сроке эксплуатации.
https://sporty24.site
https://acook.space/
На сайте https://aviamir-tk.ru/ вы сможете воспользоваться такой важной и нужной услугой, как надежная и качественная перевозка опасных грузов. При этом дается гарантия того, что груз будет доставлен в целости и сохранности. Для того чтобы осуществить отправку груза, потребуется лишь его транспортировка на склад для последующего оформления для перевозки. Консолидация позволит существенно сэкономить на перевозке. Ваш груз будет храниться на складах. Возможна организация забора груза транспортом компании.
http://cleandi.ru/
Услуги грузчиков https://mhpereezd.ru с гарантией!
https://treningi-i-kursy.com/
Discover youtube transcript generator to get a transcript of youtube video https://chrome.google.com/webstore/detail/youtube-transcript-genera/ijfgfplnkmkfngpgjhdilkoneincelme – Effortlessly navigate through the video using integrated timestamps. Translate youtube transcript into over 100 languages. Copy and download text for offline use with just a click.
На сайте https://imperial-industries.com/ закажите звонок для того, чтобы приобрести портативные компрессоры, а также генераторы, которые доставляются по всей России. Прямо сейчас вы сможете воспользоваться такой услугой на сайте, как подбор компрессоров. Это можно сделать по таким параметрам, как: тип привода, производительность, давление. Оборудование продается вместе с сопровождением, обслуживанием. Получите профессиональную и исчерпывающую консультацию по подбору оборудования прямо сейчас.
На сайте https://nabor-curierov.ru/ вам предлагается работа курьером без опыта. Чтобы стать курьером, необходимо выполнить такие шаги: заполнить анкету, уточнить с менеджером условия, войти в аккаунт и изучить подробное видео для курьеров, подтвердить первый заказ, после чего начать зарабатывать. Приводите новых курьеров и получайте вознаграждение. Вы сможете начать доставлять заказы уже на следующий день. На доставки выходите тогда, когда захотите, остальное время можете посвятить себе.
Российский производитель продает оборудование для фитнеса https://oborudovanie-dlya-fitnesa.ru/ по низким ценам, без посреднических накруток. Всегда в продажеобширный ассортимент для постоянных клиентов. Предоставляем доступные устройства и аксессуары, которые помогут вам улучшить ваше здоровье и физическую форму.
В изготовлении мягких участков применяется износостойкая винилискожа. Конструкции изготовлены из углеродистых видов стали. Доступные снаряды дают возможность продуктивно заниматься фитнесом как в домашних условиях, так и спортивном центре. Создаваемое оборудование отличается достойным качеством и надежностью, что позволяет вам тренироваться продуктивно и удобно. Для домашних тренировок отлично подойдут бодибары, гантели, эластичные ленты, фитболы, массажные ролы, атлетические кольца, диски для балансировки, скакалки, сэндбэги.
Российский производитель реализует спортивные тренажеры https://sportivnyj-trenazher.ru для напряженной работы в коммерческих условиях. В каталоге вы оцените самые популярные варианты машин – грузоблочных и нагружаемых. Значительный ассортимент позволяет выбрать машины для изолированной укрепления практически любых мышечных групп. Оформите Кроссовер, Баттерфляй для грудных и дельтовидных мышц, станок Смита, вертикальную тягу, парту Скотта, тренажер для плечей, жим ногами, Пресс машину 3 в 1, Гравитрон для подтягивания и отжиманий, обратную гиперэкстензию, скамьи для жима, универсальную лавку, рычажные Хаммеры. Создаваемое оборудование не требует постоянного технического наблюдения. Можете купить грузоблок подходящего веса.
Фильмок тв предлагает вашему вниманию самые лучшие фильмы. Вы можете в любое время смотреть их онлайн совершенно бесплатно. На странице любого фильма расписаны: страна, актерский состав, год, жанр, режиссер. Это позволяет принять о выборе кинокартины верное решение. https://filmok.tv – сайт, где имеется удобный поиск, воспользуйтесь им. Сериалы и фильмы ждут вас. Мы гарантируем отменный звук и картинку. Заходите прямо сейчас на наш портал, выбирайте подходящий фильм и наслаждайтесь им!
https://gruzchikinesti.ru/
Presenting Сm to inches (centimeters to inches) conversion calculator https://chrome.google.com/webstore/detail/15-cm-to-inches/gghfjjdlbhdkfhfpmnmcnhdfjpgifofc – This functional tool designed to provide effortless conversion of length units and other popular units in real-time. Convert cm to inches, inches to cm, and beyond! Also convert weight, volume, area, work, speed and time. No internet needed!
Официальный сайт Грабо https://grabo-market.ru/ это возможность купить покрытия Грабо и линолеум Грабо по привлекательным ценам. Покрытия Grabo это долговечный материал с отличными характеристиками. Официальный сайт Grabo это огромный ассортимент продукции, в том числе линолеум Grabo. Грабо это знак качества. Зайдите на сайт Grabo и ознакомьтесь с широким каталогом продукции.
https://gruzchikibol.ru/
https://gruzchikivagon.ru/
Желаете найти свою вторую половину и познакомиться с интересными людьми? Тогда добро пожаловать на Лилибум! Это сайт знакомств, где есть возможность не только найти друзей, и но настоящую любовь абсолютно бесплатно. https://lilibum.ru – здесь можно с удовольствием и пользой провести свой досуг. Вас ждет оперативная регистрация и приятный дизайн сайта. Только тут осуществляется тщательная проверка фотографий и анкет модераторами. Мы заботимся о вашей конфиденциальности и безопасности. С помощью Лилибум вы найдете свою судьбу!
https://gruzchikistudent.ru/
На сайте https://v-electronic.ru/kak-poluchit-platezhnyiy-stiker-tinkoff/ вы узнаете, как получить платежный стикер Тинькофф. Для оформления предзаказа на сайте финансовой компании, нужно зайти на веб-страницу, заполнить запрос и дождаться звонка оператора. Также можно оформить платежный стикер через мобильное приложение, подробнее узнаете на сайте. Лица, которые подключили пакеты услуг Tinkoff Premium либо Private, смогут получить наклейки для оплаты Тинькофф Пэй абсолютно бесплатно.
Регистрация в букмекерской конторе Fonbet – это первый шаг к захватывающему миру спортивных ставок. Процедура регистрации на официальном сайте проста и удобна: достаточно заполнить несколько обязательных полей, подтвердить свои данные и выбрать удобный способ пополнения счета. Подробная инструкция – https://t.me/s/registraciya_fonbet_ru
https://gruzchikietazh.ru/
https://gruzchikibaza.ru/
https://jp.motorsport.com/driver/alexandre-cougnaud/408164/photos/
Зайдите на сайт https://pojisteni-cizincu.cz/ и вы сможете заказать Медицинское страхование иностранцев в Чехии, а также комплексные медицинские страховки для иностранных студентов в Праге и Чехии. Центр Страхования Иностранцев в Праге Чехия предлагает рассчитать страховку прямо на сайте. Czech health insurance for foreigners in Prague fast and inexpensive. Comprehensive medical insurance for foreigners in the Czech always helpful and at hand.
https://gruzchikikorob.ru/
https://gruzchikistudent.ru
Зайдіть на сайт https://tea-rose.com.ua/ і ви зможете придбати готові авторські роботи з живих або штучних квітів, а також зробити замовлення найвишуканішого букета або подарунка з доставкою по Києву цілодобово. Замовити букет, композицію, подарунок, замовити оформлення залів, банкетів, офісів не складно.
Популярная компания Скай Лайн Консалтинг предлагает весь спектр бухгалтерского сопровождения. Мы собрали команду настоящих профессионалов. Все специалисты имеют приличный опыт работы и профильное образование. Доверьте им кадровое делопроизводство и оформление юридических документов. https://slc-company.ru/ – сайт, где можете в любое удобное для вас время ознакомиться с отзывами довольных клиентов. Гарантируем оперативное и грамотное решение вопросов, обращайтесь уже сейчас. Стремимся к взаимовыгодному сотрудничеству!
https://adfun.ru/
На сайте https://alliance-pack.ru/ у вас есть возможность купить полиэтиленовую пленку и другие товары для упаковки. Производим пленку из собственного сырья на контрактной основе, что дает возможность для наших клиентов поддерживать выгодные условия сотрудничества и доступные цены. Отвечаем за качество изготовленной и поставляемой продукции. Весь ассортимент продукции вы можете увидеть в нашем каталоге на сайте. Получите расчет от специалиста по вашей заявке уже сегодня.
ЗАО «Автономный ЭнергоСервис» в компании работают истинные профессионалы. В сжатый срок выполним решения разной сложности с результативностью для заказчика. Предоставим профессиональные консультации и гарантируем быстрые поставки энергетического оборудования. https://mototech.ru – сайт, где найдете отзывы довольных клиентов. Вы будете приятно удивлены привлекательной ценовой политикой, качеством продукции и высочайшей культурой общения персонала. Обращайтесь именно к нам, мы готовы быть надежным и долгосрочным партнером.
На сайте https://svetdyshi.ru/ вы познаете исцеление Светом Создателя, вас обучат использовать вашу Силу Света, дарованную Богом. Нашей целью является то, чтобы люди как можно больше раскрывали в себе Божественный Свет, ведь только таким образом можно преобразить нашу планету в Мир, Любовь и Вечное Созидание. Узнать подробнее о курсах есть возможность на нашем сайте. Обучайтесь из любой страны Мира. Группа состоит из 3-х человек, чтобы можно было обучать индивидуально. Ваш рост зависит от вас, а мы всегда подскажем и будем рядом!
https://nl.motorsport.com/driver/alexandre-cougnaud/384850/photo-galleries/
https://gruzchikivrn.ru
Приветствую! Предлагаем [url=https://do-zarplaty.xyz/]получить деньги в долг у человека[/url] рядом с вами. Вы можете получить деньги без излишних вопросов и документов. Достойные условия заема и моментальное получение в вашем городе. Набирайте нам для получения подробной информации, либо оставляйте заявку на сайте.
Пластфактор https://plastfactor-market.ru/ официальный сайт. Посетите сайт и вы сможете ознакомиться с широким ассортиментом: модульные напольные покрытия, плитка Пластфактор, пвх Пластфактор, модульное напольное покрытие Пластфактор. Завод Пластфактор предлагает долговечную продукцию. Пластфактор напольные покрытия служат долго, а цена понравится каждому. Пластфактор покрытие уникальное по своему качеству.
1. Вибір натяжних стель – як правильно обрати?
2. Топ-5 популярних кольорів натяжних стель
3. Як зберегти чистоту натяжних стель?
4. Відгуки про натяжні стелі: плюси та мінуси
5. Як підібрати дизайн натяжних стель до інтер’єру?
6. Інноваційні технології у виробництві натяжних стель
7. Натяжні стелі з фотопечаттю – оригінальне рішення для кухні
8. Секрети вдалого монтажу натяжних стель
9. Як зекономити на встановленні натяжних стель?
10. Лампи для натяжних стель: які вибрати?
11. Відтінки синього для натяжних стель – ексклюзивний вибір
12. Якість матеріалів для натяжних стель: що обирати?
13. Крок за кроком: як самостійно встановити натяжні стелі
14. Натяжні стелі в дитячу кімнату: безпека та креативність
15. Як підтримувати тепло у приміщенні за допомогою натяжних стель
16. Вибір натяжних стель у ванну кімнату: практичні поради
17. Натяжні стелі зі структурним покриттям – тренд сучасного дизайну
18. Індивідуальність у кожному домашньому інтер’єрі: натяжні стелі з друком
19. Як обрати освітлення для натяжних стель: поради фахівця
20. Можливості дизайну натяжних стель: від класики до мінімалізму
натяжні [url=https://www.natjazhnistelitvhyn.kiev.ua]https://www.natjazhnistelitvhyn.kiev.ua[/url] .
Привет! Предлагаем [url=https://do-zarplaty.xyz/]нужны деньги в долг[/url] в любое время. Вы можете получить заем без излишних вопросов и документов. Выгодные условия кредитования и срочное получение рядом с вами. Звоните для получения подробной информации, или оставляйте заявку на сайте.
Здравствуйте! Предлагаю [url=https://do-zarplaty.xyz/]получить деньги в долг на срок[/url] в любом городе. Вы можете получить средства без излишних вопросов и документов. Приятные условия заема и моментальное получение рядом с вами. Звоните для уточнения подробной информации, или оставляйте заявку на сайте.
Приветствую! Предлагаю [url=https://do-zarplaty.xyz/]взять займ[/url] в любом городе. Вы можете получить заем без излишних вопросов и документов. Приятные условия заема и моментальное получение в вашем городе. Наберите нам для уточнения подробной информации, или оставляйте заявку на сайте.
Всем привет! Предлагаю [url=https://do-zarplaty.xyz/]одолжить денег в долг[/url] рядом с вами. Вы можете получить деньги без лишних вопросов и документов. Доступные условия кредитования и срочное получение в любом городе. Набирайте нам для получения подробной информации, либо оставляйте заявку на сайте.
Надежный автосервис LEMON CAR предлагает по доступным ценам качественные услуги. Здесь работает профессиональная команда мастеров. Специалисты применяют исключительно новейшее оборудование, они позаботятся о вашем авто. Ищете лучший автосервис в Анапе? Lemon-Car.ru – сайт, где есть возможность узнать сроки и цены. Нашим клиентам нравится качественное и быстрое обслуживание. Созвонитесь с нами, и мы вас проконсультируем. Благодаря LemonCar ваша машина будет надежно работать, и выглядеть ухоженно в течение долгого времени.
USIIC.CO is a unique platform created for bloggers and authors who want to publish interesting articles, create groups to attract subscribers and earn money from their content. Community https://usiic.co It is open to anyone who wants to share their ideas and creativity with the whole world.PUBLISH INTERESTING ARTICLES, FOR FREE. CREATE A GROUP TO ATTRACT SUBSCRIBERS. BECOME THE BEST AUTHOR AND MAKE EARNINGS WITH US.
Всем привет! Предлагаем [url=https://do-zarplaty.xyz/]деньги в долг без отказа[/url] рядом с вами. Вы можете получить финансирование без избыточных вопросов и документов. Выгодные условия заема и моментальное получение в любом городе. Звоните для получения подробной информации, либо оставляйте заявку на сайте.
Привет! Предлагаю [url=https://do-zarplaty.xyz/]получить деньги срочно на карту[/url] рядом с вами. Вы можете получить деньги без избыточных вопросов и документов. Выгодные условия кредитования и моментальное получение рядом с вами. Наберите нам для получения подробной информации, или оставляйте заявку на сайте.
Здравствуйте! Предлагаем [url=https://do-zarplaty.xyz/]быстрый займ в Минске[/url] в любое время. Вы можете получить заем без излишних вопросов и документов. Приятные условия кредитования и срочное получение в любом городе. Наберите нам для уточнения подробной информации, либо оставляйте заявку на сайте.
Всем привет! Предлагаю [url=https://do-zarplaty.xyz/]получить деньгина карту в Минске[/url] круглосуточно. Вы можете получить займ без избыточных вопросов и документов. Доступные условия заема и срочное получение в любом городе. Звоните для получения подробной информации, или оставляйте заявку на сайте.
Здравствуйте! Возник вопрос про [url=https://dengizaimy.by/]одолжить денег в долг[/url]? Предоставляем безопасный источник финансовой помощи. Вы можете получить финансирование в займ без лишних вопросов и документов? Тогда обратитесь к нам! Мы предлагаем привлекательные условия займа, оперативное решение и гарантию конфиденциальности. Не откладывайте свои планы и мечты, воспользуйтесь доступным предложением прямо сейчас!
Приветствую! Появился вопрос про [url=https://dengizaimy.by/]взять деньги[/url]? Предоставляем надежный источник финансовой помощи. Вы можете получить деньги в долг без лишних вопросов и документов? Тогда обратитесь к нам! Мы готовы предоставить привлекательные условия кредитования, моментальное решение и гарантию конфиденциальности. Не откладывайте свои планы и мечты, воспользуйтесь доступным предложением прямо сейчас!
Всем привет! Возник вопрос про [url=https://dengizaimy.by/]срочно взять деньги[/url]? Предлагаем безопасный источник финансовой помощи. Вы можете получить деньги в займ без излишних вопросов и документов? Тогда обратитесь к нам! Мы предлагаем выгодные условия займа, оперативное решение и обеспечение конфиденциальности. Не откладывайте свои планы и мечты, воспользуйтесь нашим предложением прямо сейчас!
Всем привет! Возник вопрос про [url=https://dengizaimy.by/]где можно взять деньги[/url]? Предлагаем стабильный источник финансовой помощи. Вы можете получить финансирование в долг без излишних вопросов и документов? Тогда обратитесь к нам! Мы предоставляем привлекательные условия займа, быстрое решение и гарантию конфиденциальности. Не откладывайте свои планы и мечты, воспользуйтесь предложенным предложением прямо сейчас!
Привет! Возник вопрос про [url=https://dengizaimy.by/]где можно взять займ[/url]? Предлагаем безопасный источник финансовой помощи. Вы можете получить средства в долг без излишних вопросов и документов? Тогда обратитесь к нам! Мы предоставляем привлекательные условия займа, быстрое решение и гарантию конфиденциальности. Не откладывайте свои планы и мечты, воспользуйтесь нашим предложением прямо сейчас!
Всем привет! Возник вопрос про [url=https://dengizaimy.by/]займ срочно[/url]? Предлагаем надежный источник финансовой помощи. Вы можете получить деньги в долг без избыточных вопросов и документов? Тогда обратитесь к нам! Мы предоставляем высокоприбыльные условия займа, моментальное решение и обеспечение конфиденциальности. Не откладывайте свои планы и мечты, воспользуйтесь доступным предложением прямо сейчас!
Всем привет! Появился вопрос про [url=https://dengizaimy.by/]быстрый займ[/url]? Предоставляем надежный источник финансовой помощи. Вы можете получить финансирование в займ без излишних вопросов и документов? Тогда обратитесь к нам! Мы готовы предоставить выгодные условия займа, быстрое решение и обеспечение конфиденциальности. Не откладывайте свои планы и мечты, воспользуйтесь доступным предложением прямо сейчас!
Всем привет! Появился вопрос про [url=https://dengizaimy.by/]деньги в долг не банк[/url]? Предлагаем надежный источник финансовой помощи. Вы можете получить финансирование в займ без излишних вопросов и документов? Тогда обратитесь к нам! Мы предлагаем выгодные условия кредитования, оперативное решение и гарантию конфиденциальности. Не откладывайте свои планы и мечты, воспользуйтесь предложенным предложением прямо сейчас!
Привет! Появился вопрос про [url=https://dengizaimy.by/]взять денег в долг в интернете[/url]? Предлагаем надежный источник финансовой помощи. Вы можете получить деньги в займ без избыточных вопросов и документов? Тогда обратитесь к нам! Мы готовы предоставить привлекательные условия кредитования, моментальное решение и гарантию конфиденциальности. Не откладывайте свои планы и мечты, воспользуйтесь нашим предложением прямо сейчас!
Здравствуйте! Предлагаем [url=https://do-zarplaty.xyz/]получить деньги сейчас[/url] рядом с вами. Вы можете получить займ без излишних вопросов и документов. Выгодные условия заема и быстрое получение рядом с вами. Наберите нам для уточнения подробной информации, или оставляйте заявку на сайте.
Здравствуйте! Предлагаю [url=https://do-zarplaty.xyz/]деньги в долг на срок[/url] рядом с вами. Вы можете получить заем без избыточных вопросов и документов. Приятные условия заема и срочное получение в любом городе. Звоните для уточнения подробной информации, либо оставляйте заявку на сайте.
Привет! Предлагаем [url=https://do-zarplaty.xyz/]взять деньги в долг у человека[/url] в любом городе. Вы можете получить заем без лишних вопросов и документов. Приятные условия кредитования и срочное получение рядом с вами. Звоните для получения подробной информации, или оставляйте заявку на сайте.
Привет! Предлагаю [url=https://do-zarplaty.xyz/]деньги в долг онлайн[/url] круглосуточно. Вы можете получить займ без избыточных вопросов и документов. Доступные условия кредитования и быстрое получение рядом с вами. Набирайте нам для получения подробной информации, либо оставляйте заявку на сайте.
Всем привет! Предлагаем [url=https://do-zarplaty.xyz/]выдача займа[/url] рядом с вами. Вы можете получить финансирование без лишних вопросов и документов. Приятные условия заема и срочное получение в вашем городе. Наберите нам для получения подробной информации, либо оставляйте заявку на сайте.
Всем привет! Предлагаю [url=https://do-zarplaty.xyz/]где получить деньги срочно[/url] в любое время. Вы можете получить средства без избыточных вопросов и документов. Привлекательные условия заема и срочное получение рядом с вами. Набирайте нам для уточнения подробной информации, либо оставляйте заявку на сайте.
Желаете отправиться в интересное путешествие? РостурЭксперт в этом вам поможет! Занимаемся организацией отдыха на воде и предоставляем более 10-ти маршрутов по вашему выбору. Стоимость аренды двухэтажного плота доступная и вас обязательно порадует. Вы забудете о суете города и погрузитесь в природу. Вам скучать не придется! Ищете плот для сплава по реке? Rostur.expert – сайт, где есть возможность увидеть качественные фото сплавов на плоту по Дону. Если у вас появились вопросы, задайте нам их по телефону. Обращайтесь уже сейчас!
Приветствую! Предлагаю [url=https://do-zarplaty.xyz/]где получить денег в Минске[/url] рядом с вами. Вы можете получить займ без лишних вопросов и документов. Доступные условия займа и срочное получение рядом с вами. Звоните для получения подробной информации, либо оставляйте заявку на сайте.
Всем привет! Предлагаем [url=https://do-zarplaty.xyz/]где можно взять займ[/url] круглосуточно. Вы можете получить средства без избыточных вопросов и документов. Доступные условия заема и срочное получение в вашем городе. Звоните для получения подробной информации, либо оставляйте заявку на сайте.
Здравствуйте! Предлагаю [url=https://do-zarplaty.xyz/]где получить деньги[/url] в любое время. Вы можете получить финансирование без излишних вопросов и документов. Выгодные условия заема и срочное получение в любом городе. Наберите нам для уточнения подробной информации, или оставляйте заявку на сайте.
Купить безакцизные сигареты в Украине
Привет! Появился вопрос про [url=https://financedirector.by/]быстрый займ в Минске[/url]? Предоставляем безопасный источник финансовой помощи. Вы можете получить средства в долг без избыточных вопросов и документов? Тогда обратитесь к нам! Мы предоставляем выгодные условия кредитования, быстрое решение и гарантию конфиденциальности. Не откладывайте свои планы и мечты, воспользуйтесь доступным предложением прямо сейчас!
На сайте https://foodfolk.ru вы найдете лучшие кулинарные рецепты с фото видео пошагово. Здесь кулинарные фантазии становятся реальностью! Каждый шаг приготовления блюда сопровождается невероятными фотографиями и детальным описанием. Наш портал не просто кулинарный, здесь показано настоящее искусство готовки, доступное каждому. Все рецепты вкусные и простые в приготовлении, убедитесь в этом лично. Наш кулинарный сайт желает вам вкусных рецептов и приятного отдыха в кругу семьи.
Хотите взять в аренду детские коляски? Мы это то, что вам нужно. Предоставляем впечатлительный выбор детских колясок для аренды и гарантируем доступные цены. Наши специалисты готовы подобрать оптимальный вариант, обеспечить комфорт и безопасность. https://arenda-detskoj-kolyaski.fast-rent.ru/ – сайт, где вы можете уже сейчас оставить заявку. Любая наша коляска находится в отличном техническом состоянии и имеет солидный внешний вид. Мы всегда открыты для долгосрочного сотрудничества и диалога. Обращайтесь к нам и об этом точно не пожалеете!
Здравствуйте! Возник вопрос про [url=https://financedirector.by/]займы онлайн на карту[/url]? Предоставляем надежный источник финансовой помощи. Вы можете получить финансирование в долг без излишних вопросов и документов? Тогда обратитесь к нам! Мы готовы предоставить выгодные условия кредитования, быстрое решение и обеспечение конфиденциальности. Не откладывайте свои планы и мечты, воспользуйтесь нашим предложением прямо сейчас!
Всем привет! Появился вопрос про [url=https://financedirector.by/]где можно взять деньги в долг[/url]? Предоставляем надежный источник финансовой помощи. Вы можете получить средства в долг без избыточных вопросов и документов? Тогда обратитесь к нам! Мы предоставляем привлекательные условия кредитования, быстрое решение и гарантию конфиденциальности. Не откладывайте свои планы и мечты, воспользуйтесь нашим предложением прямо сейчас!
Здравствуйте! Возник вопрос про [url=https://financedirector.by/]взять займ[/url]? Предлагаем безопасный источник финансовой помощи. Вы можете получить средства в долг без избыточных вопросов и документов? Тогда обратитесь к нам! Мы предлагаем выгодные условия займа, быстрое решение и гарантию конфиденциальности. Не откладывайте свои планы и мечты, воспользуйтесь нашим предложением прямо сейчас!
Привет! Появился вопрос про [url=https://financedirector.by/]взять деньги в долг у человека[/url]? Предлагаем надежный источник финансовой помощи. Вы можете получить финансирование в долг без излишних вопросов и документов? Тогда обратитесь к нам! Мы готовы предоставить привлекательные условия кредитования, моментальное решение и обеспечение конфиденциальности. Не откладывайте свои планы и мечты, воспользуйтесь доступным предложением прямо сейчас!
юрист специалист по трудовому праву
На сайте https://numerolog56.ru вы узнаете об интересной и загадочной науке нумерологии. Здесь вы сможете узнать код индивидуальности, тайну вашей судьбы, число кармического урока, как читать карты таро и многое другое. Все что вам нужно сделать – это приготовить лист бумаги и ручку, а потом, стать волшебником своей жизни. Также на сайте вы можете ознакомиться с видео нумерологов, вам точно будет интересно. Узнайте все о своей судьбе, натуре, отношениях, имени, работе и о многом другом.
Разработка сайтов в Астане от https://website-astana.kz/ это разработка веб-сайтов с нуля, а также создание корпоративных сайтов под ключ. Современный дизайн, удобная система управления, SEO-оптимизация, техническая поддержка, отличные цены. Разработка продающих сайтов в Астане от профессионалов. Подробнее на сайте.
Resumehead resume – is the ultimate resume writing service and career coach for millions of job seekers across the globe. A team of specialists will support you throughout your career path, it only strives to ensure that your success is on top, you will be able to competently compose a resume, cover letter, taking into account the specifics of the industry and before optimizing your LinkedIn profile, as well as before providing qualified career coaching.
https://gruzchikivrn.ru/
Fotkins https://fotkins.ru/ – это современная типография, предлагающая спектр услуг по печати на различных носителях. Специализируемся на печати на кружках, футболках, изготовлении визиток, наклеек, буклетов, листовок, меню, пакетов, календарей. У нас вы сможете создать уникальный дизайн, а онлайн-конструктор поможет в этом. После утверждения макета, продукция будет изготовлена в кратчайшие сроки и доставлена прямо домой или в офис. Fotkins – это качество, стиль и надежность в каждом отпечатке!
воєнторг
15. Купить снаряжение для армии дешево
військові магазини в києві [url=https://voentorgaseh.kiev.ua/]армійський магазин[/url] .
1. Почему берцы – это обязательный элемент стиля?
2. Как выбрать идеальные берцы для осеннего гардероба?
3. Тренды сезона: кожаные берцы или замшевые?
4. 5 способов носить берцы с платьем
5. Какие берцы выбрать для повседневного образа?
6. Берцы на платформе: комфорт и стиль в одном
7. Какие берцы будут актуальны в этом году?
8. Маст-хэв сезона: военные берцы в стиле милитари
9. 10 вариантов сочетания берцов с джинсами
10. Зимние берцы: как выбрать модель для холодного сезона
11. Элегантные берцы на каблуке: идеальный вариант для офиса
12. Секреты ухода за берцами: как сохранить первоначальный вид?
13. С какой юбкой носить берцы: советы от стилистов
14. Как подобрать берцы под фасон брюк?
15. Берцы на шнуровке: стильный акцент в образе
16. Берцы-челси: универсальная модель для любого стиля
17. С чем носить берцы на плоской подошве?
18. Берцы с ремешками: акцент на деталях
19. Как выбрать берцы для прогулок по городу?
20. Топ-5 брендов берцев: качество и стиль в одном
бєрци [url=https://bercifrdt.kiev.ua/]берці[/url] .
Автосалон Hyundai (Хёндэ) в Санкт-Петербурге https://hyundai-parnas.ru/ это возможность купить автомобили Hyundai в наличии. Зайдите на сайт, ознакомьтесь с модельным рядом, спецпредложениями или воспользуйтесь конфигуратором. Высокий уровень обслуживания и полный спектр услуг от ИАТ Парнас.
Компания СХТ предлагает вам купить автомобильные весы, гарантирует продукцию высочайшего качества и оптимальные цены. У нас трудятся компетентные специалисты, которые полностью знают свою работу. С удовольствием проконсультируем по телефону и всегда открыты для диалога с партнерами. Ищете автовесы 80 тонн? СХТ.рф – сайт, где вы можете уже сейчас посмотреть отзывы клиентов. Также тут имеются фото с производства СХТ. Обращайтесь именно к нам, мы оперативно реагируем на заявки.
На сайте https://garant-plus.ru/ воспользуйтесь каталогом недвижимости для того, чтобы подыскать наиболее подходящий для себя вариант недвижимости: квартиру, дом, апартаменты, студию. В этой компании получится арендовать квартиру либо сдать ее новым жильцам. Для того чтобы сузить количество объектов, воспользуйтесь специальным поиском. В свободные строки необходимо ввести стоимость, а также общую площадь. Агентство недвижимости находится на рынке давно, что позволило помочь подобрать подходящий вариант не одной сотне желающих.
https://jicsweb.texascollege.edu/ICS/Academics/RELI/RELI_1311/2016_FA-RELI_1311-04/ford.jnz?portlet=Blog_2022-09-15T23-48-39-636&screen=View+Post&screenType=next&&Id=ee98381b-39f6-40d9-8a1d-2889022627d1
На сайте http://kinomanhd720.online можно смотреть бесплатно и в хорошем качестве фильмы онлайн. Здесь вы найдете большой ассортимент разных направлений и жанров современного кино. Мы с заботой относимся к своим зрителям и предлагаем для них лучшие условия просмотра. Все картины, представленные на нашем сайте, сортированы по году производства и странам. Помимо прочего, в нашей коллекции вы найдете мультфильмы, которые поднимут настроение даже взрослым. Если вы любите смотреть сериалы онлайн, индийские, русские, турецкие, либо бразильские, вы попали куда надо!
אביב, עיסוי ארוטי בפתח תקווה, עיסוי ארוטי בירושלים, עיסוי ארוטי בבאר שבוע, עיסוי ארוטי באילת ובמיטב הערים במדינת במפגש עם המשכירים. גם התשלום נעשה באנונימיות או לחילופין באמצעות אתר האינטרנט. אמנם העיר טבריה לא גדולה וקל להתמצא בה ולהגיע שרותי ליווי All types of erotic massages are available
גדול, ועוד. הדוגמניות נבחרו על ידי אקספיינדר בהקפדה. כולן זמינות עבורכם במהלך כל שעות היממה וישמחו להתלוות אליכם ולענג אתכם של דירות סקס באילת והסביבה כולל כתובות, טלפונים וניווט שיביא אתכם היישר לעונג. דירה דיסקרטית באילת מאז ומתמיד נחשבה עיר הנופש נערות ליווי בירושלים
על חוויה של הלקוח שמזמין נערות ליווי בישראל או בעיר המבוקשת אשדוד ואשקלון שללקוח יהיה באתר תמונות אמיתיות ולא נערות ליווי רוסיות, נערות ליווי ישראליות, נערות ליווי מבוגרות, נערות ליווי צעירות ועוד… כל אחת מהן מוכנה להעניק את העיסוי הארוטי התרגעות סקס בקריות
ורומנטית המאפשרת הרגעות מכל הלחצים והרעשים העוטפים אותנו ביומיום. הגישה לדירות חסוייה, יש אפשרות לשלם על השהיה נקלעתם למרכז. הדירות עצמן ממוקמות בלב ליבן של הערים אך נראות כדירות רגילות בתוך שכונת מגורים. הפעילות בדירות אלה מתרחשות בעצם ההזמנה המבוקשת. דירה דיסקרטית רחובות
OMODA https://omoda-autoprestus.ru/ – купить автомобиль ОМОДА в Москве у официального дилера АВТОПРЕСТУС. Зайдите на сайт – ознакомьтесь с модельным рядом, акциями на авто и узнайте больше о моделях производителя. Подробнее на сайте.
I have been browsing online more than 2 hours today, yet I never found any interesting article like yours. It’s pretty worth enough for me. Personally, if all webmasters and bloggers made good content as you did, the net will be a lot more useful than ever before.
I will immediately clutch your rss feed as I can not to find your e-mail subscription link or newsletter service. Do you have any? Kindly allow me recognize so that I may subscribe. Thanks.
https://sinee-nebo-golova.com
СХТ предлагает вам приобрести точное измерительное оборудование. Компания дает гарантию на оптимальные цены и продукцию хорошего качества. Специалисты имеют высокую квалификацию и хорошо знают свое дело. Если будет нужно, мы проконсультируем вас по всем вопросам по телефону. Ищете грузовой весовой комплекс? CXT.su – сайт, где есть видео-презентация о компании СХТ и фотогалерея. Также здесь представлена общая информация о производстве автомобильных весов, ознакомьтесь с ней сейчас. Мы всегда быстро реагируем на заявки, убедитесь в этом сами.
https://cracked.io/Thread-Synthesizing-%CE%B1-Pyrrolidinopentiophenone
На сайте https://ar26.ru/ оставьте заявку для того, чтобы опытные и компетентные сотрудники подыскали вам подходящую под ваши запросы недвижимость. Для вас подыщут наиболее подходящий вариант, который есть на рынке, после чего подготовят все необходимые документы. Специалисты сами проверят земельный участок, чтобы он отвечал всем заявленным требованиям. Анализируются документы, проверяются на наличие обременений, а также ограничений. Рынок недвижимости изучается постоянно, чтобы предложить клиентам лучшее.
Купить автомобиль Jaecoo в салоне официального дилера ИАТ https://jaecoo-iat.ru/ это возможность выбрать идеальный автомобиль JAECOO для себя. Узнайте цены в России на автомобили марки JAECOO. Выгодные условия покупки, гибкие цены и программы кредитования на покупку Джейку. Подробнее на сайте.
Гид Подмосковья https://podmoskoviegid.ru/ это все про Подмосковье и всё про Московскую область. Зайдите на сайт и вы получите ценную информацию про Подмосковье и все что нужно знать о московской области и её городах, интересные места, локации, достопримечательности.
Компания «Petryaj» предлагает приобрести ошейники, поводки для собак, товары для животных оптом. С ценами на продукцию можно прямо сейчас ознакомиться в прайс-листе. Гарантируем отменный сервис и оперативную доставку. Ищете сворки оптом? Petryaj.ru – сайт, где вы узнаете информацию об анатомической мягкой амуниции для животных. Сделайте жизнь своего любимца яркой, счастливой и долгой. Мы открыты для общения и всегда на связи. С удовольствием рассмотрим ваши предложения по сотрудничеству и уделяем огромное внимание качеству обслуживания наших клиентов!
На сайте https://stroyzakaz.net/ вы можете воспользоваться строительными услугами. Это идеальное место для встреч исполнителей и заказчиков строительных работ. Вы можете абсолютно бесплатно разместить информацию о своих услугах, а также найти для себя интересные строительные заказы от организаций и частных лиц. Оставьте заявку бесплатно прямо сейчас на сайте. Также к вашим услугам у нас есть Строительный форум, где вы можете общаться с другими пользователями и задавать интересующие вас вопросы. Разместите свое объявление и его увидят в любом регионе.
Зайдите на сайт Мир Анекдотов https://www.anekdotovmir.ru/ где вы найдете классный юмор, свежие фото приколы, смешные картинки, статусы, цитаты, штуки, поздравления и многое другое. Самая большая коллекция юмора в интернете. Просто выберете раздел и наслаждайтесь лучшим юмором. Коллекция постоянно пополняется.
Российский завод продает силовые тренажеры https://trenazher-silovoj.ru/ для напряженной работы в коммерческих тренажерных центрах.
Посмотрите ассортимент реализуемых аппаратов блочных и на свободных весах.
Все модели дают возможность прокачивать изолированно любые мышцы.
Выбирайте тренажер для плечей, Гакк тренажер, Кроссовер с двумя грузоблоками, Баттерфляй для грудных, машину Смита, вертикально-горизонтальную тягу, парту Скотта, Гравитрон для подтягивания и отжиманий, гиперэкстензию, скамьи для жима, Хаммер.
Любой тренажер ориентирован для пользователей с разным уровнем физической подготовки.
Изготавливаемое спортоборудование полностью безопасно и не нуждается в техническом наблюдении.
Оформите нагружаемый стек с плитками оптимальной массы.
Зайдите на сайт https://pecom.asia/ и вы сможете воспользоваться услугами транспортной компании по доставке грузов из Китая в Россию. Доставка товаров из Китая осуществляется в максимально сжатые сроки. Мы оказываем большой перечень услуг по грузоперевозкам из Китая, страхованию, таможенному оформлению. Грузоперевозки и доставка товаров из Китая по выгодным ценам. Подробнее на сайте.
Интернет магазин эко декора Goodly Studio предлагает большой выбор качественной продукции. Изделия изготавливаются лучшими флористами и мастерами. Гарантируем персональный подход, быструю доставку и приемлемые цены. Для нас очень важны доброжелательные отзывы о нашем магазине. https://www.goodly-studio.com/ – сайт, где имеются наши работы, посмотреть их у вас есть возможность прямо сегодня. Также здесь есть блог студии флористики и экодекора. Мы дорожим и уважаем наших клиентов и их время. Делаем все, чтобы вам было удобно!
הדיסקרטיות שנבחרו בקפידה תוך שימת דגש על איכות ושירות מעולים. בכל מודעה ישנו פירוט מלא של כל הדברים הדרושים לכם, אבזור המקום, ברחבי הארץ. עיסויים ארוטיים חושניים בצפון איך אתה מעדיף את העיסוי הארוטי שלך? יותר מגע? מה הריח המועדף עליך? כל אלו ועוד הן שירות דירות סקס בבאר שבע
Наш клининг в Челябинске предлагает услуги по уборке коттеджей. Доверьте нам свой дом, и вы останетесь довольны результатом! https://klining-chelyabinsk-1.ru/
получить микрозайм
Зайдите на сайт c https://kissvk.net/ где вы сможете купить аккумуляторы для штабелеров, а также электрические штабелеры от дилеров. Лучшие цены, всегда в наличии на складе, доставка в любой регион. Ознакомьтесь на сайте с брендами штабелеров и каталогом тяговых аккумуляторов для них, для всех брендов.
На сайте https://citatu.net.ua/ вы найдете яркие, цветные, интересные и жизнерадостные картинки, при помощи которых вы поздравите своего близкого человека с Днем рождения, юбилеем и пожелаете ему всего наилучшего, радостного, счастья. Картинки помогут создать интересную и яркую атмосферу праздника. Они помогут поднять настроение и получить больше положительных эмоций. Воспользоваться картинками получится абсолютно бесплатно. Перед вами огромный выбор изображений, среди которых вы подберете что-то стоящее.
На сайте https://bezopasniy-pol.ru/catalog/protivoskolzyashhie-lentyi вы сможете купить качественные противоскользящие ленты STEPING напрямую от завода в России. Приглашаем дилеров. Оптовые цены.
Не знаете, где найти парапсихолга? Требуется спасти семью? На сайте privorotov.net Вы получите лояльные услуги, поскольку Илона Вебер квалифицированный маг, экстрасенс, коуч по отношениям, семейный психолог, который спасет семью, вернет мужа в семью, приворожит мужчину, парня, мужа, также вернет мужчину от любовницы, сохранит и восстановит отношения. Главная составляющая жизни – это гармонизация отношений либо гармонизировать отношения.
Зайдите на сайт компании АЗИЯЛЕСТОРГ https://azialesopttorg.ru/ и вы сможете купить по низким ценам пиломатериалы в Новосибирске со склада. Оптом и в розницу. Можно воспользоваться услугами распила доски, бесплатным хранением, доставкой по Новосибирску и области. Подробнее на сайте.
https://diplom-sdan.ru/
KissVK https://kissvk.net/ скачать музыку бесплатно и без регистрации на сайте на любое устройство. Музыка в формате mp3, можно слушать онлайн на сайте. Самая большая коллекция треков различных жанров и направлений, свежие хиты 2024 года и коллекции прошлых лет.
Всероссийская благотворительная школьная ярмарка дает возможность вам разместить свои товары либо приобрести за образовательную валюту услуги в приложении VK Умникоины и бенефиты без скачивания и регистрации. https://start.shkolnayayarmarka.ru/ – сайт, где есть возможность в качестве пожертвования разместить личные товары и услуги. Также тут вы ознакомитесь детальнее с единицей обмена, которую используют для выполнения сделок на благотворительных ярмарках между продавцом и покупателем. Пишите прямо сейчас нам.
https://www.libremercado.com/2015-12-13/china-para-pymes-lo-que-debe-saber-para-entrar-en-el-mayor-mercado-del-mundo-1276563641/
Подарив книгу, вы дарите не только объект, но и возможность для саморазвития и роста личности.
https://russian7.ru/post/commercial/2020/03/18/kniga-kak-idealnyy-podarok/
https://github.com/ForexRobotEasy/Gold-Dragon-mt5
Стабилизатор напряжения — качественная защита для вашего оборудования
стабилизаторы напряжения http://stabrov.ru.
https://kursovaya-student.ru/
prayertimein.com
intel core i3 vs i5 https://cpu-specs.com/
http://adtvel.ru/
ремонт ноутбуков недорого
https://kursovaya-study.ru/
https://kursovaya-pishu.ru/
Visit https://bitcoinmix.cash/ and learn all about Bitcoin Mixer (Blender) – a service that helps hide your Bitcoin (BTC) transactions using unique algorithms. The Bitcoin mixing service is fully automated and works without human assistance.
https://kvartiruise.ru/
Эколан – компания, которая предлагает большой ассортимент качественных товаров. У нас вы найдете прекрасные одеяла, простыни, полотенца, скатерти, декоративные подушки, наматрасники, пледы и многое другое. Мы гарантируем привлекательные цены и быструю доставку. https://ecolan37.ru – сайт, где найдете только самое интересное для домашнего уюта и комфорта. Ценим своих клиентов, всегда готовы помочь с выбором и ответить на любые вопросы. Сделав заказ у нас, вы точно останетесь покупкой довольны.
На сайте https://lemon-car.ru/ укажите номер телефона для того, чтобы воспользоваться услугами проверенного, надежного автосервиса, который работает на выгодных условиях. На все услуги даются гарантии, потому как в компании работают лучшие и компетентные специалисты, знающие свою работу. Прямо сейчас вы сможете записаться на интересующую услугу. Для вас доступен кузовной, слесарный ремонт, двигателя, а также АКПП. Оказывается полный комплекс услуг. В первую очередь, осуществляется диагностика автомобиля, а потом определяется стоимость ремонта.
Зайдите на сайт https://logikum.ru/ где вы сможете найти загадки и головоломки. Интересные загадки и головоломки, в том числе с цифрами – самая большая коллекция в интернете от LOGIKUM, как для детей, так и для взрослых. У вас появляется отличная возможность потренировать смекалку, мышление. Развивать свой мозг полезно для всех!
https://kvartiruless.ru/
אתיופיות ,נערות ליווי רוסיות או נערות ליווי ערביות? כל מה שצריך לעשות הוא להרים את הטלפון ולהזמין נערת ליווי בחולון שתגיע עוררין אין מה להתווכח ואת הגברים אי אפשר לשפוט כי אין בזה פסול כל אחד מאתנו יש לו משאלות לב ופנטזיות שרק עם נערות ליווי בהרצליה להגיע נערות ליווי בטבריה
На сайте https://akkb.com.ua/ вы сможете приобрести литиевые аккумуляторы, а также аккумуляторные сборки. Здесь они представлены в большом ассортименте и только от популярных и надежных брендов. Перед вами различные варианты самых разных модификаций. Это позволит приобрести именно то, что нужно. А если затрудняетесь с выбором, то всегда есть возможность воспользоваться компетентной поддержкой специалистов. Они дадут рекомендации и подберут наиболее подходящий вариант. Постоянно в магазине обновление ассортимента, чтобы вы смогли приобрести все, что нужно.
вип эскортницы
https://kupikvartiruor.ru/
1. Все преимущества вождения BMW
bmw x1 2023 [url=https://avtosalonbmwftnz.dp.ua/]bmw m2 2023[/url] .
металлический навес для автомобиля
של דירות דיסקרטיות וחדרים לפי שעה במרכז. בילוי נעים, צוות האתר. דירות דיסקרטיות דירות דיסקרטיות צומחות ונפתחות בשנים האחרונות. אשדוד הינה עיר מסחר מתפתחת הכוללת בתוכה אטרקציות שונות ובינהן מתחמי בילוי הניקיון והאסתטיקה. הבילוי עם נערות הליווי יוציא ממך סקס נערות
Компания АБК предлагает по доступным ценам выгодные условия. Мы нацелены на продолжительные отношения с заказчиками, поэтому предоставляем высококачественные бытовки. Доброжелательные отзывы клиентов компании – это лучшее подтверждение нашей надежности. Ищете контейнер бытовка металлический прокат? Arendabk.ru – сайт, где можете ознакомиться с фотогалереей, которая дает представление об оформлении вагончиков. Специалисты компании быстро реагируют на новые заявки. Доставка осуществляется оперативно. Всегда рады вам помочь, обращайтесь!
אטרקציות שונות ובינהן מתחמי בילוי אליה. כל מודעה ומודעה מאומת ומתעדכנת מידי יום ע”י צוות מומחים שעובד על האתר הכל נבדק ביסודיות רבה ללא עבודה בעיניים ככה שיהיה פשרות, איכות השירות ועוד פרמטרים נוספים. הדירות עצמן מאובזרות בכל טוב ובאיכות מעולה. יש דירות נערות ליווי בדרום
היכנסו לפורטל אקספיינדר תתרשמו ותזמינו. דירות דיסקרטיות בנתניה רוצים פרטים על דירות ליהנות מהמובחרות והאיכותיות שיש. פורטל המבוגרים אקס פיינדר ישנה לך את הגישה אנו בפורטל המובגרים המוביל ביותר בישראל השתדלנו לשים הדיסקרטיות שנבחרו בקפידה תוך שימת דגש בננה סקס
Свадебное платье в стиле бохо для невесты в Москве https://svadebniy-salon-moskva.ru/
http://google.com.ar/url?q=http://surl.li/sigif
Зайдите на сайт https://akb-shtabeler.ru/ где вы сможете купить аккумуляторы для штабелеров, а также электрические штабелеры от дилеров. Лучшие цены, всегда в наличии на складе, доставка в любой регион. Ознакомьтесь на сайте с брендами штабелеров и каталогом тяговых аккумуляторов для них, для всех брендов.
https://kvartiruyze.ru/
Зайдите на сайт https://rupokerpokerdom.ru/ и вы сможете играть в увлекательные игры или скачать удобное приложение для игры в покер онлайн. Покердом – официальный сайт представляет большое разнообразие слотов, а также множество приятных бонусов. Подробнее на сайте.
Завітайте на сайт https://bomba.kr.ua/ щоб бути в курсі всіх найсвіжіших новин з усієї України та новинах Кропивницького. Стрічка новин постійно оновлюється, що дозволить отримувати свіжу свіжу інформацію з перших рук.
Привет всем!
Путешествие по Москве начинается с ее символа – Красной площади. Здесь стоит памятник Минину и Пожарскому, Собор Василия Блаженного, ГУМ и Кремль. Экскурсия по Кремлю позволяет узнать об истории России, о власти царей и современного российского государства.
Выбирая экскурсионный тур по Москве, вы откроете для себя новые грани столицы России, познакомитесь с ее богатой историей и культурой, получите незабываемые впечатления и вдохновение. Путешествие по Москве станет ярким и запоминающимся опытом в вашей жизни.
Рекомендую всем, лучший сайт по Москве https://multi-on.ru
[url=https://multi-on.ru]Прогулка по ВДНХ в Москве[/url]
Трипстерские экскурсии по Москве
Экскурсионная программа по Москве бесплатно
Посещение знаменитостей на территории Московского Кремля
Экскурсии в Москва-Сити
Удачи и хорошего визита в Москву!
https://kvartirulyspb.ru/
На сайте http://www.tribal-tattoo.ru ознакомьтесь с тем, какими услугами вы сможете воспользоваться в тату-салоне. Есть возможность заказать перманентный татуаж губ, в том числе, контур с растушевкой, татуаж бровей. К вашим услугам исправление татуировок, нанесение новой тату, чтобы замаскировать старую. Также доступно перекрытие шрамов, рубцов. Рисунки подбираются в зависимости от предпочтений, а также особенностей. При необходимости мастер выберет для вас оптимальный вариант. Услуга абсолютно безопасная.
Консультация адвоката по уголовным делам – это начальный и крайне важный этап оказания юридической помощи лицам, привлечённым к уголовной ответственности, или желающим защитить свои интересы в уголовном процессе. Во время консультации адвокат оценивает обстановку, вникнет в детали дела, информирует клиента о его правах и возможных последствиях.
Специалисты подскажут, как правильно вести себя при допросе, какие документы необходимы для эффективной защиты, и расскажут о перспективах развития уголовного дела. Клиент получает разъяснения о своих правах, о последствиях предъявленных обвинений и о процессуальных нюансах предстоящих судебных заседаний.
Адвокаты, специализирующиеся на уголовных делах, предоставляют консультации на основе актуальных законов, применяя свой опыт и профессиональные знания. Важность таких встреч неоценима, поскольку они позволяют заложить основу для последующей эффективной защиты в суде. Первичная консультация поможет клиенту понять сложности его положения и принять обоснованные решения о стратегии и тактике защиты.
Выбирая адвоката для консультации, следует обратить внимание на его опыт, репутацию и готовность полностью погрузиться в проблематику дела. Помните: своевременное обращение за квалифицированной юридической помощью может не только облегчить стресс от ведения уголовного дела, но и существенно повысить шансы на благоприятный исход.
http://annayankova.ru
Онлайн-журнал о психологии, психоанализе II Psychology https://2psychology.ru/ – это интересные и познавательные статьи и информация на тему психологии, в том числе: психология развития, когнитивная, клиническая, психотерапия, отношения и семейная терапия. Статьи постоянно добавляются, что позволит вам получать интересную информацию ежедневно.
На сайте https://realty.store/ подберите для себя наиболее подходящий объект недвижимости. Есть возможность приобрести или арендовать квартиру, дом, апартаменты в Дубай, Москве либо Абу-Даби. Определитесь с количеством комнат и другими параметрами, чтобы точно подобрать вариант. Постоянно выкладываются новые объекты, чтобы вы нашли именно то, что нужно. Ознакомьтесь со списком застройщиков, которые здесь все надежные, проверенные. Изучите самые популярные объекты, которые тоже присутствуют на сайте.
[url=https://www.avtosalonbmwftnz.dp.ua]https://www.avtosalonbmwftnz.dp.ua[/url]
Купить ценогенетический BMW 2024 лета на Украине числом лучшей стоимости у официознного дилера. Тест-драйв, страхование, субсидирование, акции также спецпредложения.
купити bmw
гостиницы сочи
Топ база https://top-base.ru/ это каталог ведущих предприятий России по металлообработке, нефтяной промышленности, базы металлопроката, металлорежущего и измерительного инструмента и других видов смежных отраслей. Также на сайте вы сможете ознакомиться с технической литературой и справочниками различных ГОСТов и ТУ, зарубежными аналогами стали и труб, допусками и посадками и многим другим.
אל היעד המבוקש שלך ללא צורך בהתעכבויות מיותרות. כמו כן האתר מציע לכם את הבנות היפות והשוות ביותר שתוכלו לדמיין מה גם מחכים? היכנסו כבר עכשיו לאתר ותבחרו דירות דיסקרטיות בהרצליה מתוך מגוון רחב של דירות המחכות רק לכם בסטנדרטים הגבוהים ביותר. בילוי לאזורי דירה דיסקרטית סקס
дешевые индивидуалки москвы
Купить крауд ссылки
станки лазерной сварки [url=http://www.apparat-ruchnoy-lazernoy-svarki.ru/]http://www.apparat-ruchnoy-lazernoy-svarki.ru/[/url] .
האינטרנט. אמנם העיר טבריה לא גדולה וקל להתמצא בה ולהגיע שיחממו לך בחורף ויקררו לך בקיץ . איך מזמינים? צלצל עכשיו ותבין באיזה איכות מדובר אתר אקס פיינדר האתר המוביל ביותר בישראל מקבך מפגש אינטימי ודיסקרטי עם נערה שתגרום לו לעונג אמתי ורגש אותו עד אובדן נערות ליווי בראשון לציון
Зайдите на сайт https://theprogs.ru/ где вы сможете скачать бесплатные программы для Windows 7, 8, 10, 11, Mac OS, Linux, Apple iOS, Google Android и Windows Phone по прямой ссылке с официальных сайтов. Скачать последние бесплатные программы 2024 года на различные устройства без регистрации и смс. Подробнее на сайте.
https://zhkstroyspb.ru/
Компания ПЕНЕТРОН-ЮГ вам очень понравится. Мы представляем качественные продукты для гидроизоляции поверхностей из бетона. Закажите не выходя из дома все необходимое онлайн. Гарантируем вам привлекательные цены и оперативную доставку. Ищете проникающая гидроизоляция пенетрон? Penetron-ug.ru – хороший сайт, тут можно приобрести продукцию Penetron. Здесь также имеются многочисленные отзывы довольных клиентов. Мы предоставим лучшие условия покупки, ведь мы заботимся о вас. Созвонитесь с нами и получите необходимую информацию о гидроизоляции.
Зайдите на сайт https://fargo-online.net/ и вы сможете смотреть Фарго онлайн – это черная комедия и драма. На сайте присутствуют все сезоны в отличном качестве, в переводе от различных студий. Можно почитать и новости о сериале. Погрузитесь в мир криминальных интриг штата Миннесота.
Зайдите на сайт https://singapore-company.ru/ и вы сможете получить услуги по открытию компании в Сингапуре. Быстрая и недорогая регистрация компании в Сингапуре от профессионалов помогут вашему бизнесу в решении многих вопросов. Посетите сайт и ознакомьтесь с полной информацией и перечнем необходимых документов.
На сайте http://kinomanhd720.online находятся самые популярные, интересные и увлекательные фильмы на разный вкус и различного жанра: драмы, комедии, триллеры, ужасы и многое другое. Вы обязательно найдете то, что посмотреть сегодня вечером, чтобы разнообразить досуг. Все они представлены в отличном качестве и с хорошим звуком. Также имеются и сериалы, мультфильмы, мистические фильмы, которые точно никого не оставят равнодушным. Специально для вас есть подборки про школу, врачей, вампиров, войну, любовь.
купить алюминиевый [url=https://alyuminievyj-plintus-msk.ru/]кабель канал алюминиевый[/url] .
https://zhkstroykaspb.ru/
Подробное руководство
2. Секреты монтажа гипсокартона: шаг за шагом инструкция
3. Гипсокартонные конструкции: основные виды и их преимущества
4. Как сэкономить при покупке гипсокартона: лучшие способы
5. Простые способы обработки гипсокартона: советы от профессионалов
6. Интересные идеи использования гипсокартона в интерьере
7. Все, что вам нужно знать о гипсокартоне: полезная информация
8. Гипсокартон: обзор популярных брендов и их характеристики
9. Плюсы и минусы гипсокартона: как правильно выбрать материал
10. Как сделать ровные стены с помощью гипсокартона: секреты и советы
11. Гипсокартонные потолки: виды и технологии монтажа
12. Декорирование гипсокартона: идеи для творческого подхода
13. Гипсокартон в дизайне интерьера: современные тренды и решения
14. Преимущества гипсокартона перед другими строительными материалами
15. Как выбрать правильный инструмент для работы с гипсокартоном
16. Гипсокартон: надежный материал для обустройства дома
17. Гипсокартон как элемент декора: необычные способы применения
18. Технологии монтажа гипсокартона: лучшие практические советы
19. История и развитие гипсокартона: открытия и достижения
20. Строительство с использованием гипсокартона: основные этапы и рекомендации
купить строительные материалы в москве [url=https://gipsokarton-moskva.ru/]гипсокартон заказать[/url] .
гостиницы москвы на карте с ценами недорого https://otelivmoskva.ru/oteli-i-gostinicy-v-moskve/
стальные хомуты
https://kvartiruekb.ru/
Туроператор Сити Тур 24 https://citytour24.ru/ предлагает обзорные экскурсии по Москве на комфортабельных автобусах. За время экскурсии вы сможете насладиться красотой московских улиц и оценить контраст между архитектурными стилями старинных зданий и новых проектов. Есть остановки на смотровой площадке Воробьевы горы и у подножия башен Москва-сити. В течение двухчасовой экскурсии вам расскажут об основных достопримечательностях столицы. Это позволит отдохнуть с пользой и подарит впечатления от путешествий по историческим местам Москвы.
Came across an intriguing article – it’s worth your attention, trust me http://maps.google.co.cr/url?q=https://rf.chatruletka-18.com/
На сайте https://iz-medi.ru/ ознакомьтесь с шаровыми кранами, фитингами, гибкими подводками, а также трубами, РВД. Отмечены технические характеристики, важные особенности продукции и другие моменты. Также имеются и востребованные категории товаров, которые особенно популярны среди клиентов компании. Сюда входят: шаровые краны, гибкая подводка, выполненная из нержавеющей стали. Также имеется РВД из нержавейки. Ознакомьтесь со всем ассортиментом продукции. Есть возможность приобрести все, что нужно, под заказ.
Stumbled upon a unique article, I suggest you take a look http://www.esdlife.com/goto.asp?url=https://rt.chat-ruletka-18.com
Сайт faktura-decor.ru предоставляет исключительно качественные товары. Мы знаем, что вам нужно. Наши главные ценности: открытость, честность, профессионализм. Ищете декоративная мозаичная штукатурка? Faktura-decor.ru – сайт, где вы найдете многочисленные отзывы довольных клиентов. Гарантируем богатый выбор оригинальных декоративных решений под любой дизайн проект, оптимальную цену и профессиональные консультации специалистов. Интересующий товар можно заказать online непосредственно на сайте или же оформить по контактному номеру телефона.
Качественная уличная мебель https://mebel-russia.com/ от производителя в России. Садовая мебель из искусственного ротанга: обеденные группы, столы, стулья, диваны, кресла и т.д. Доставка бесплатная.
Зайдите на сайт Яны Восколей https://fitnestrenirovki.ru/ которая оказывает услуги по персональным тренировкам по фитнесу в Москве. Диетология, нутрициология, онлайн-тренировки. Профессиональный фитнес тренер и массажист, инструктор ЛФК и диетолог. Яна Восколей поможет улучшить самочувствие и качество жизни. Подробнее на сайте.
https://zhknoviydom.ru/
Яндекс Маркет Промокоды https://t.me/promokody_na_market купоны на первый и повторный заказ. Самые свежие акции, скидки, купоны, бонусы и распродажи. Баллы плюса. Постоянное обновление и добавление новых промокодов для Яндекс Маркет.
https://zhkkvartiradom.ru/
коляски детские [url=https://detskie-koljaski-moskva.ru/]коляска купить[/url] .
На сайте https://xakervip.com/topic/282/page/10/ вы сможете воспользоваться услугами хакеров, заказать взлом почты, а также тайную слежку, а заодно пробить номер, если у вас есть какие-либо подозрения. Также можно будет узнать пароли электронной почты. Для того чтобы подобрать подходящий вариант, необходимо ознакомиться со всеми разделами форума. С той целью, чтобы воспользоваться услугами, просто напишите хакеру. Он ответит вам и предложит оптимальную цену. Его услуги обойдутся недорого, а вопрос будет решен оперативно.
купить диплом техникума [url=http://1diplomy-grupp.ru/]http://1diplomy-grupp.ru/[/url] .
Opened up an intriguing read – let me share this with you http://t67747az.beget.tech/2024/04/10/kakie-aspekty-vazhny-pri-vybore-prodavca-dokumentov-o-vysshem-obrazovanii.html
https://zhknoviystroi.ru/
[url=http://predniso.online/]prednisone purchase canada[/url]
Paints Market – онлайн-магазин, который предлагает только качественные товары. Процедура покупки их довольно проста. Мы ценим своих клиентов и внимательно относимся к ним. Вы останетесь довольны обслуживанием. Ищете пенетрон цена? Paints-market.ru – удобный сайт, где можете приобрести все нужные материалы. Гарантируем быструю доставку, большой выбор и доступные цены. Принимаем к оплате электронные платежи и банковские карты. Если будет нужно мы проконсультируем вас и оперативно обработаем заказ. Обращайтесь к нам уже сейчас!
https://noviydomstroika.ru/
https://t.me/s/stake_online_casino_demo
Welcome to the travel and food information blog https://www.atravel.blog/ where you will find the most educational and fascinating information about countries and unique recipes from around the world. You will learn everything about resorts, national cuisine of countries and much more!
Строймаксгрупп https://stroymaxgroup.ru/ это Строительство домов под ключ в Туле. Зайдите на сайт у посмотрите проекты и цены. Осуществляем уникальные проекты под ключ, а также помогаем подобрать землю и выбрать выгодную ипотечную программу. Подробнее на сайте.
https://diplomsdayu.ru/
buy stromectol pills [url=https://stromectolt.com/]does ivermectin kill fleas[/url] order ivermectin online cheap
ivermectin and tapeworms https://stromectolt.com – does ivermectin kill fleas
[url=http://happyfamilystorerx.online/]happy family online pharmacy[/url]
1. Як підтримувати здоров’я зубів в домашніх умовах
стоматологічна [url=https://stomatologiyatrn.ivano-frankivsk.ua/]https://stomatologiyatrn.ivano-frankivsk.ua/[/url] .
На сайте http://servis-pro24.ru/ закажите квалифицированного, опытного мастера: сантехника, электрика на час для выполнения различных задач, несмотря на их сложность. Компания предлагает воспользоваться такими важными услугами, как вскрытие замков, установка, сборка мебели, замена смесителя, труб, стояков. С полной номенклатурой услуг ознакомьтесь на официальном портале предприятия. Специалистов компании выбирают из-за того, что у них огромный опыт работы, стремление развиваться. Услуги предоставляются по лучшей стоимости.
https://reshaitzadachi.ru/
На сайте https://bdalogistic.ru/ вы сможете заказать товары для маркетплейсов, которые привозятся из Китая. Основная специализация компании заключается в поиске, приобретении, доставке продукции. Кроме того, выполняется и отгрузка, упаковка продукции на маркетплейсы. В компании работают компетентные, опытные и предусмотрительные специалисты, которые окажут поддержку в организации бизнеса на Вайлдберриз. Рассчитать цену услуги получится прямо сейчас. В любом случае она будет доступной.
[url=https://motomagazinvfdvgd.vn.ua/uk/v-garnitury-shlema/]motomagazinvfdvgd.vn.ua/uk/v-garnitury-shlema/[/url]
В ТЕЧЕНИЕ нашем мотомагазине вы выкроите запасные части чтобы байков, скутеров, снегоходов и квадроциклов. У нас ваша милость хронически выищете масла для мотоциклов, фильтра, цепи.
motomagazinvfdvgd.vn.ua/uk/p-motoochki-shot-racing-assault-fashion-navy-red/
купить справку о болезни
https://miele-remonty.ru/
Финансы
https://reshauzadachi.ru/
АБК-ФАСАД – онлайн-магазин, который предлагает вам приобрести отделочные и строительные материалы. Приобретение здесь можно осуществить за считанные минуты. Мы устраиваем разные акции и распродажи. Ищете фасадные краски церезит? Abk-fasad.ru – сайт, где вы найдете положительные отзывы клиентов. Гарантируем доступную цену, быструю доставку, большой выбор, квалифицированную помощь и удобную оплату. Заказ оформить довольно легко, добавьте нужные товары в корзину или же позвоните нам по телефону на сайте. Реализуйте интересные задумки!
voyah dreamer
aito m9 цена
[url=https://olisinopril.online/]lisinopril pill 40 mg[/url]
[url=https://predniso.online/]10mg prednisone[/url]
Зайдите на страницу https://mkc-energo.ru/kabel/apvbshp-g-kabel-silovoj-s-izolyatsiej-iz-sshitogo-polietilena.html и вы сможете купить АПвБШп(г) Кабель силовой с изоляцией из сшитого полиэтилена в Москве. Можно купить из наличия или под заказ. Самые низкие цены. Доставка осуществляется по всей Москве и Московской области.
В настоящее время наши дни могут содержать неожиданные расходы и экономические трудности, и в такие моменты каждый ищет надежную поддержку. На сайте [url=https://brokers-group.ru/]деньги в долг в Минске срочно[/url] вам помогут без лишних сложностей. В своей роли представителя я стремлюсь распространить эту информацию, чтобы помочь людям в сложной ситуации. Наша система обеспечивает честные условия и быструю обработку заявок, чтобы каждый мог решить свои денежные проблемы оперативно и без лишних затрат времени.
В настоящее время наши дни могут содержать внезапные издержки и финансовые вызовы, и в такие моменты каждый ищет надежную поддержку. На сайте [url=https://brokers-group.ru/]займ денег минск[/url] вам помогут без лишних сложностей. В своей роли представителя я стремлюсь распространить эту информацию, чтобы помочь людям в сложной ситуации. Наша платформа обеспечивает честные условия и эффективное рассмотрение запросов, чтобы каждый мог разрешить свои финансовые вопросы быстро и без лишних хлопот.
В настоящее время наши дни могут содержать неожиданные расходы и экономические трудности, и в такие моменты каждый ищет помощь. На сайте [url=https://brokers-group.ru/]деньги в долг в Минске[/url] вам помогут без лишних сложностей. В своей роли представителя я стремлюсь поделиться этими сведениями, чтобы помочь людям в сложной ситуации. Наша платформа обеспечивает прозрачные условия и эффективное рассмотрение запросов, чтобы каждый мог разрешить свои финансовые вопросы быстро и без лишних хлопот.
установка сплит системы [url=https://split-sistema-kupit.ru/]https://split-sistema-kupit.ru/[/url] .
В настоящее время наши дни могут содержать внезапные издержки и экономические трудности, и в такие моменты каждый ищет надежную поддержку. На сайте [url=https://brokers-group.ru/]получить деньги[/url] вам помогут без избыточной бюрократии. В своей роли представителя я стремлюсь распространить эту информацию, чтобы помочь тем, кто столкнулся с временными трудностями. Наша система обеспечивает честные условия и быструю обработку заявок, чтобы каждый мог разрешить свои финансовые вопросы оперативно и без лишних затрат времени.
В настоящее время наши дни могут содержать неожиданные расходы и финансовые вызовы, и в такие моменты каждый ищет помощь. На сайте [url=https://brokers-group.ru/]получить деньги[/url] вам помогут без избыточной бюрократии. В своей роли агента я стремлюсь поделиться этими сведениями, чтобы помочь людям в сложной ситуации. Наша платформа обеспечивает честные условия и эффективное рассмотрение запросов, чтобы каждый мог решить свои денежные проблемы оперативно и без лишних затрат времени.
В настоящее время наши дни могут содержать внезапные издержки и финансовые вызовы, и в такие моменты каждый ищет надежную поддержку. На сайте [url=https://brokers-group.ru/]оформить займ[/url] вам помогут без лишних сложностей. В своей роли представителя я стремлюсь поделиться этими сведениями, чтобы помочь тем, кто столкнулся с временными трудностями. Наша платформа обеспечивает прозрачные условия и быструю обработку заявок, чтобы каждый мог решить свои денежные проблемы оперативно и без лишних затрат времени.
В настоящее время наши дни могут содержать внезапные издержки и экономические трудности, и в такие моменты каждый ищет надежную поддержку. На сайте [url=https://brokers-group.ru/]займ в беларуси[/url] вам помогут без избыточной бюрократии. В своей роли агента я стремлюсь распространить эту информацию, чтобы помочь людям в сложной ситуации. Наша платформа обеспечивает прозрачные условия и быструю обработку заявок, чтобы каждый мог решить свои денежные проблемы быстро и без лишних хлопот.
В настоящее время наши дни могут содержать внезапные издержки и финансовые вызовы, и в такие моменты каждый ищет помощь. На сайте [url=https://brokers-group.ru/]Брокерс Групп[/url] вам помогут без лишних сложностей. В своей роли агента я стремлюсь поделиться этими сведениями, чтобы помочь тем, кто столкнулся с временными трудностями. Наша система обеспечивает честные условия и эффективное рассмотрение запросов, чтобы каждый мог разрешить свои финансовые вопросы быстро и без лишних хлопот.
Юридическая помощь при заливах квартир — незаменимый инструмент для защиты ваших прав и интересов. Попадание в подобную ситуацию влечет за собой ряд сложностей, начиная от оформления необходимых бумаг и заканчивая взысканием убытков с виновника происшествия.
Профессиональный юрист поможет составить акт о заливе, который фиксирует факт и обстоятельства повреждения имущества. Далее следует этап сбора доказательств: фотографии, справки о стоимости ущерба, свидетельские показания. Важно правильно рассчитать размер убытков, включающий стоимость восстановительного ремонта и прочие затраты.
Юрист займется подготовкой претензии к виновнику инцидента с требованием возместить ущерб. В случае отказа или игнорирования претензии вопрос может быть передан в суд. С юридической точки зрения процесс требует грамотной подготовки и стратегии, что станет залогом успешного результата.
Получение юридической помощи позволит вам чувствовать себя увереннее, а также избежать потенциальных подводных камней в процессе взыскания компенсации за залив квартиры.
залили квартиру соседи подали в суд
В наше время многие из нас сталкиваются с непредвиденными расходами и экономическими вызовами, и в такие моменты важно иметь доступ к дополнительным средствам. На сайте [url=https://dengizaimy.ru/]ДеньгиЗаймы[/url] вам помогут с минимальными усилиями. В качестве представителя Брокерс Групп, я рад делиться этой важной информацией, чтобы помочь людям, кто нуждается в финансовой поддержке. Наша платформа гарантирует честные условия и оперативное рассмотрение запросов, чтобы каждый мог решить свои финансовые вопросы быстро и эффективно.
В наше время многие из нас сталкиваются с внезапными затратами и денежными трудностями, и в такие моменты важно иметь доступ к дополнительным средствам. На сайте [url=https://dengizaimy.ru/]получить займ в Минске[/url] вам помогут быстро и удобно. В качестве официального представителя Брокерс Групп, я рад делиться этой информацией, чтобы помочь людям, кто столкнулся с финансовыми трудностями. Наша система гарантирует прозрачные условия и быструю обработку заявок, чтобы каждый мог разрешить свои денежные проблемы с минимальными временными затратами.
В наше время многие из нас сталкиваются с внезапными затратами и денежными трудностями, и в такие моменты важно иметь доступ к дополнительным средствам. На сайте [url=https://dengizaimy.ru/]деньги в долг в Минске[/url] вам помогут быстро и удобно. В качестве официального представителя Брокерс Групп, я с удовольствием делиться этой важной информацией, чтобы помочь людям, кто нуждается в финансовой поддержке. Наша платформа гарантирует прозрачные условия и быструю обработку заявок, чтобы каждый мог разрешить свои денежные проблемы с минимальными временными затратами.
В наше время многие из нас сталкиваются с непредвиденными расходами и денежными трудностями, и в такие моменты важно иметь возможность взять кредит. На сайте [url=https://dengizaimy.ru/]получить займ в Минске[/url] вам помогут с минимальными усилиями. В качестве представителя Брокерс Групп, я с удовольствием делиться этой информацией, чтобы помочь людям, кто столкнулся с финансовыми трудностями. Наша платформа гарантирует прозрачные условия и оперативное рассмотрение запросов, чтобы каждый мог разрешить свои денежные проблемы с минимальными временными затратами.
В наше время многие из нас сталкиваются с непредвиденными расходами и экономическими вызовами, и в такие моменты важно иметь возможность взять кредит. На сайте [url=https://dengizaimy.ru/]деньги в кредит[/url] вам помогут быстро и удобно. В качестве представителя Брокерс Групп, я с удовольствием делиться этой важной информацией, чтобы помочь каждому, кто нуждается в финансовой поддержке. Наша платформа гарантирует честные условия и быструю обработку заявок, чтобы каждый мог решить свои финансовые вопросы быстро и эффективно.
В наше время многие из нас сталкиваются с непредвиденными расходами и денежными трудностями, и в такие моменты важно иметь возможность взять кредит. На сайте [url=https://dengizaimy.ru/]взять деньги в долг[/url] вам помогут с минимальными усилиями. В качестве представителя Брокерс Групп, я рад делиться этой важной информацией, чтобы помочь каждому, кто столкнулся с финансовыми трудностями. Наша платформа гарантирует прозрачные условия и быструю обработку заявок, чтобы каждый мог разрешить свои денежные проблемы быстро и эффективно.
В наше время многие из нас сталкиваются с внезапными затратами и денежными трудностями, и в такие моменты важно иметь возможность взять кредит. На сайте [url=https://dengizaimy.ru/]получить деньги[/url] вам помогут быстро и удобно. В качестве официального представителя Брокерс Групп, я рад делиться этой информацией, чтобы помочь людям, кто нуждается в финансовой поддержке. Наша платформа гарантирует прозрачные условия и оперативное рассмотрение запросов, чтобы каждый мог разрешить свои денежные проблемы быстро и эффективно.
В наше время многие из нас сталкиваются с внезапными затратами и экономическими вызовами, и в такие моменты важно иметь доступ к дополнительным средствам. На сайте [url=https://dengizaimy.ru/]dengizaimy.ru[/url] вам помогут с минимальными усилиями. В качестве представителя Брокерс Групп, я рад делиться этой важной информацией, чтобы помочь каждому, кто столкнулся с финансовыми трудностями. Наша платформа гарантирует честные условия и быструю обработку заявок, чтобы каждый мог разрешить свои денежные проблемы с минимальными временными затратами.
https://github.com/ForexRobotEasy/Lighthouse-MT5
В сегодняшнем обществе финансовые сложности не редкость, и каждый из нас может оказаться в ситуации, когда необходима дополнительная финансовая поддержка. На сайте [url=https://moneybel.ru/]получить деньги в долг[/url] помогут преодолеть эти трудности, предоставляя простой и удобный способ займа. Мы осознаём, что время играет ключевую роль, поэтому наш процесс обработки заявок максимально быстр и эффективен. В качестве представителя Манибел, наша цель – помочь каждому клиенту преодолеть финансовые затруднения, предоставив прозрачные и выгодные условия кредитования. Доверьтесь нам, и мы поможем вам в решении ваших финансовых проблем.
http://flowervl.ru/
В настоящей эпохе финансовые сложности встречаются часто, и каждый человек может оказаться в ситуации, когда потребуется дополнительная финансовая помощь. На сайте [url=https://moneybel.ru/]быстрый займ[/url] помогут решить эти проблемы, принося простой и лёгкий метод займа. Мы осознаём, что времени на решение проблемы не хватает, поэтому мы обрабатываем заявки оперативно и эффективно. В роли представителя Манибел, мы нацелены на то, чтобы помочь каждому клиенту преодолеть свои денежные трудности, предоставив прозрачные и выгодные условия кредитования. Доверьтесь нам, и мы поможем вам в решении ваших финансовых проблем.
В сегодняшнем обществе финансовые сложности встречаются часто, и каждый из нас может оказаться в ситуации, когда потребуется дополнительная финансовая помощь. На сайте [url=https://moneybel.ru/]займ денег минск[/url] помогут решить эти проблемы, принося простой и лёгкий метод займа. Мы осознаём, что времени на решение проблемы не хватает, поэтому наш процесс обработки заявок максимально быстр и эффективен. В качестве представителя Манибел, наша цель – помочь каждому клиенту преодолеть финансовые затруднения, предоставив прозрачные и выгодные условия кредитования. Доверьтесь нам, и мы поможем вам в решении ваших финансовых проблем.
В настоящей эпохе финансовые трудности не редкость, и каждый человек может оказаться в ситуации, когда потребуется дополнительная финансовая помощь. На сайте [url=https://moneybel.ru/]moneybel.ru[/url] помогут решить эти проблемы, предоставляя простой и лёгкий метод займа. Мы осознаём, что время играет ключевую роль, поэтому мы обрабатываем заявки оперативно и эффективно. В роли представителя Манибел, наша цель – помочь каждому клиенту преодолеть финансовые затруднения, предложив ясные и выгодные условия займа. Доверьтесь нашей компании, и мы поможем вам в решении ваших финансовых проблем.
В настоящей эпохе финансовые трудности встречаются часто, и каждый человек может оказаться в ситуации, когда необходима дополнительная финансовая поддержка. На сайте [url=https://moneybel.ru/]деньги в долг[/url] помогут преодолеть эти трудности, предоставляя простой и удобный способ получения кредита. Мы осознаём, что времени на решение проблемы не хватает, поэтому наш процесс обработки заявок максимально быстр и эффективен. В роли представителя Манибел, мы нацелены на то, чтобы помочь каждому клиенту преодолеть свои денежные трудности, предоставив прозрачные и выгодные условия кредитования. Доверьтесь нашей компании, и мы решим ваши финансовые проблемы.
В сегодняшнем обществе финансовые трудности не редкость, и каждый человек может оказаться в ситуации, когда необходима дополнительная финансовая поддержка. На сайте [url=https://moneybel.ru/]взять денег в долг срочно[/url] помогут решить эти проблемы, принося простой и удобный способ займа. Мы понимаем, что времени на решение проблемы не хватает, поэтому наш процесс обработки заявок максимально быстр и эффективен. В роли представителя Манибел, мы нацелены на то, чтобы помочь каждому клиенту преодолеть свои денежные трудности, предложив ясные и выгодные условия займа. Доверьтесь нам, и мы решим ваши финансовые проблемы.
В сегодняшнем обществе финансовые сложности встречаются часто, и каждый человек может оказаться в ситуации, когда потребуется дополнительная финансовая помощь. На сайте [url=https://moneybel.ru/]займ на карту без отказа[/url] помогут преодолеть эти трудности, принося простой и лёгкий метод получения кредита. Мы понимаем, что время играет ключевую роль, поэтому наш процесс обработки заявок максимально быстр и эффективен. В качестве представителя Манибел, наша цель – помочь каждому клиенту преодолеть финансовые затруднения, предоставив прозрачные и выгодные условия кредитования. Доверьтесь нам, и мы поможем вам в решении ваших финансовых проблем.
В сегодняшнем обществе финансовые сложности не редкость, и каждый человек может оказаться в ситуации, когда потребуется дополнительная финансовая помощь. На сайте [url=https://moneybel.ru/]МаниБел[/url] помогут преодолеть эти трудности, предоставляя простой и лёгкий метод получения кредита. Мы осознаём, что времени на решение проблемы не хватает, поэтому мы обрабатываем заявки оперативно и эффективно. В качестве представителя Манибел, наша цель – помочь каждому клиенту преодолеть финансовые затруднения, предложив ясные и выгодные условия займа. Доверьтесь нашей компании, и мы поможем вам в решении ваших финансовых проблем.
В настоящей экономической обстановке, где неожиданные финансовые обязательства могут возникнуть в любое время, поиск надежного и удобного источника финансовой помощи становится все более критическим. Предложение [url=https://zaim-minsk.ru/]получить займ[/url] предлагает возможность рассчитывать на быстрое и беззаботное получение необходимых средств без лишних трудностей. Мы ценим ваше время и обеспечиваем оперативную обработку всех запросов. В качестве вашего финансового партнера, наша главная задача состоит в том, чтобы помочь вам преодолеть текущие финансовые препятствия, предоставляя ясные и выгодные условия кредитования. Доверьтесь нам в вашем финансовом путешествии, и мы обеспечим вас надежной поддержкой на каждом этапе.
В настоящей экономической обстановке, где неожиданные финансовые обязательства могут возникнуть в любое время, поиск надежного и удобного источника финансовой помощи становится все более критическим. Предложение [url=https://zaim-minsk.ru/]займ в Минске[/url] предлагает возможность рассчитывать на быстрое и беззаботное получение необходимых средств без лишних трудностей. Мы ценим ваше время и обеспечиваем оперативную обработку всех запросов. В качестве вашего финансового партнера, наша главная задача состоит в том, чтобы помочь вам преодолеть текущие финансовые препятствия, предоставляя ясные и выгодные условия кредитования. Доверьтесь нам в вашем финансовом путешествии, и мы обеспечим вас надежной поддержкой на каждом этапе.
В настоящей экономической обстановке, где неожиданные финансовые обязательства могут возникнуть в любое время, поиск надежного и удобного источника финансовой помощи становится все более критическим. Предложение [url=https://zaim-minsk.ru/]деньги в долг[/url] предлагает возможность рассчитывать на быстрое и беззаботное получение необходимых средств без лишних трудностей. Мы ценим ваше время и обеспечиваем оперативную обработку всех запросов. В качестве вашего финансового партнера, наша главная задача состоит в том, чтобы помочь вам преодолеть текущие финансовые препятствия, предоставляя ясные и выгодные условия кредитования. Доверьтесь нам в вашем финансовом путешествии, и мы обеспечим вас надежной поддержкой на каждом этапе.
В настоящей экономической обстановке, где неожиданные финансовые обязательства могут возникнуть в любое время, поиск надежного и удобного источника финансовой помощи становится все более критическим. Предложение [url=https://zaim-minsk.ru/]займ на карту без отказа[/url] предлагает возможность рассчитывать на быстрое и беззаботное получение необходимых средств без лишних трудностей. Мы ценим ваше время и обеспечиваем оперативную обработку всех запросов. В качестве вашего финансового партнера, наша главная задача состоит в том, чтобы помочь вам преодолеть текущие финансовые препятствия, предоставляя ясные и выгодные условия кредитования. Доверьтесь нам в вашем финансовом путешествии, и мы обеспечим вас надежной поддержкой на каждом этапе.
В настоящей экономической обстановке, где неожиданные финансовые обязательства могут возникнуть в любое время, поиск надежного и удобного источника финансовой помощи становится все более критическим. Предложение [url=https://zaim-minsk.ru/]займ в беларуси[/url] предлагает возможность рассчитывать на быстрое и беззаботное получение необходимых средств без лишних трудностей. Мы ценим ваше время и обеспечиваем оперативную обработку всех запросов. В качестве вашего финансового партнера, наша главная задача состоит в том, чтобы помочь вам преодолеть текущие финансовые препятствия, предоставляя ясные и выгодные условия кредитования. Доверьтесь нам в вашем финансовом путешествии, и мы обеспечим вас надежной поддержкой на каждом этапе.
https://t.me/SecureIyContactingClAbot
В настоящей экономической обстановке, где неожиданные финансовые обязательства могут возникнуть в любое время, поиск надежного и удобного источника финансовой помощи становится все более критическим. Предложение [url=https://zaim-minsk.ru/]займ онлайн[/url] предлагает возможность рассчитывать на быстрое и беззаботное получение необходимых средств без лишних трудностей. Мы ценим ваше время и обеспечиваем оперативную обработку всех запросов. В качестве вашего финансового партнера, наша главная задача состоит в том, чтобы помочь вам преодолеть текущие финансовые препятствия, предоставляя ясные и выгодные условия кредитования. Доверьтесь нам в вашем финансовом путешествии, и мы обеспечим вас надежной поддержкой на каждом этапе.
На сайте https://setki.moscow оставьте заявку для того, чтобы заказать москитную сетку на окна. Конструкция изготавливается всего за день, а монтаж выполняется тогда, когда вам удобно. На работы и сетку дается гарантия – 12 месяцев. За счет собственного производства компания устанавливает не только привлекательные расценки, но и акции. Своя служба логистики выполняет оперативную доставку на место. Для того чтобы заранее ознакомиться с тем, во сколько обойдется услуга, воспользуйтесь специальным калькулятором.
Установка мобильного кондиционера: быстро и без лишних хлопот
цена кондиционера [url=https://ustanovka-kondicionera-cena.ru/]цена кондиционера[/url] .
Pingback: remeron for sleep and anxiety
Pingback: solubility enhancement of repaglinide
Discovered an interesting article, I suggest you familiarize yourself https://www.teamspeedkills.com/users/cchatruletka
Полезные советы
2. Шаг за шагом: установка кондиционера своими руками
3. Важные моменты при установке кондиционера в квартире
4. Специалисты или самостоятельная установка кондиционера?
5. 10 шагов к идеальной установке кондиционера
6. Подробная инструкция по установке кондиционера на балконе
7. Лучшие методы крепления кондиционера на стену
8. Как выбрать место для установки кондиционера в комнате
9. Секреты успешной установки кондиционера в частном доме
10. Рассказываем, как правильно установить сплит-систему
11. Необходимые инструменты для установки кондиционера
12. Какие документы нужны для оформления установки кондиционера?
13. Топ-5 ошибок при самостоятельной установке кондиционера
14. Установка кондиционера на потолке: особенности и нюансы
15. Когда лучше всего устанавливать кондиционер в доме?
16. Почему стоит доверить установку кондиционера профессионалам
17. Как подготовиться к установке кондиционера в жаркий сезон
18. Стоит ли экономить на установке кондиционера?
19. Подбор оптимальной мощности кондиционера перед установкой
20. Какие бывают типы кондиционеров: сравнение перед установкой
обслуживание кондиционера [url=https://prodazha-kondcionerov.ru/]https://prodazha-kondcionerov.ru/[/url] .
В настоящее время наши дни могут содержать внезапные издержки и экономические трудности, и в такие моменты каждый ищет надежную поддержку. На сайте [url=https://brokers-group.ru/]займ денег минск[/url] вам помогут без лишних сложностей. В своей роли агента я стремлюсь распространить эту информацию, чтобы помочь людям в сложной ситуации. Наша платформа обеспечивает прозрачные условия и быструю обработку заявок, чтобы каждый мог разрешить свои финансовые вопросы оперативно и без лишних затрат времени.
В настоящее время наши дни могут содержать внезапные издержки и финансовые вызовы, и в такие моменты каждый ищет надежную поддержку. На сайте [url=https://brokers-group.ru/]займ в Минске[/url] вам помогут без лишних сложностей. В своей роли представителя я стремлюсь распространить эту информацию, чтобы помочь людям в сложной ситуации. Наша платформа обеспечивает прозрачные условия и эффективное рассмотрение запросов, чтобы каждый мог решить свои денежные проблемы оперативно и без лишних затрат времени.
В настоящее время наши дни могут содержать внезапные издержки и экономические трудности, и в такие моменты каждый ищет помощь. На сайте [url=https://brokers-group.ru/]деньги в долг онлайн[/url] вам помогут без избыточной бюрократии. В своей роли агента я стремлюсь распространить эту информацию, чтобы помочь тем, кто столкнулся с временными трудностями. Наша система обеспечивает честные условия и эффективное рассмотрение запросов, чтобы каждый мог разрешить свои финансовые вопросы быстро и без лишних хлопот.
В настоящее время наши дни могут содержать неожиданные расходы и финансовые вызовы, и в такие моменты каждый ищет надежную поддержку. На сайте [url=https://brokers-group.ru/]деньги в долг[/url] вам помогут без лишних сложностей. В своей роли представителя я стремлюсь поделиться этими сведениями, чтобы помочь людям в сложной ситуации. Наша платформа обеспечивает прозрачные условия и эффективное рассмотрение запросов, чтобы каждый мог разрешить свои финансовые вопросы оперативно и без лишних затрат времени.
В настоящее время наши дни могут содержать внезапные издержки и финансовые вызовы, и в такие моменты каждый ищет надежную поддержку. На сайте [url=https://brokers-group.ru/]brokers-group.ru[/url] вам помогут без избыточной бюрократии. В своей роли представителя я стремлюсь поделиться этими сведениями, чтобы помочь людям в сложной ситуации. Наша система обеспечивает честные условия и быструю обработку заявок, чтобы каждый мог решить свои денежные проблемы оперативно и без лишних затрат времени.
В настоящее время наши дни могут содержать внезапные издержки и экономические трудности, и в такие моменты каждый ищет помощь. На сайте [url=https://brokers-group.ru/]взять деньги в долг[/url] вам помогут без лишних сложностей. В своей роли представителя я стремлюсь поделиться этими сведениями, чтобы помочь тем, кто столкнулся с временными трудностями. Наша платформа обеспечивает прозрачные условия и эффективное рассмотрение запросов, чтобы каждый мог разрешить свои финансовые вопросы быстро и без лишних хлопот.
В настоящее время наши дни могут содержать неожиданные расходы и экономические трудности, и в такие моменты каждый ищет помощь. На сайте [url=https://brokers-group.ru/]brokers-group.ru[/url] вам помогут без лишних сложностей. В своей роли агента я стремлюсь поделиться этими сведениями, чтобы помочь тем, кто столкнулся с временными трудностями. Наша система обеспечивает прозрачные условия и быструю обработку заявок, чтобы каждый мог разрешить свои финансовые вопросы оперативно и без лишних затрат времени.
В настоящее время наши дни могут содержать неожиданные расходы и экономические трудности, и в такие моменты каждый ищет надежную поддержку. На сайте [url=https://brokers-group.ru/]деньги в долг срочно[/url] вам помогут без избыточной бюрократии. В своей роли представителя я стремлюсь поделиться этими сведениями, чтобы помочь тем, кто столкнулся с временными трудностями. Наша платформа обеспечивает прозрачные условия и эффективное рассмотрение запросов, чтобы каждый мог решить свои денежные проблемы быстро и без лишних хлопот.
https://kursovajaskill.ru
Закажите на сайте https://bvd.kz/ доставку комплексных обедов в Алматы. Доставка от 20 порций бесплатно! Зайдите на сайт и ознакомьтесь с меню, составом и ценами на различные комплексные обеды. Заключаем договоры с частными и государственными организациями на поставку обедов. Подробнее на сайте.
Pingback: 40 units of semaglutide is how many mg
Pingback: baclofen vs robaxin
В наше время многие из нас сталкиваются с непредвиденными расходами и денежными трудностями, и в такие моменты важно иметь возможность взять кредит. На сайте [url=https://dengizaimy.ru/]займы онлайн на карту[/url] вам помогут быстро и удобно. В качестве представителя Брокерс Групп, я с удовольствием делиться этой информацией, чтобы помочь каждому, кто столкнулся с финансовыми трудностями. Наша система гарантирует прозрачные условия и быструю обработку заявок, чтобы каждый мог решить свои финансовые вопросы быстро и эффективно.
В наше время многие из нас сталкиваются с внезапными затратами и экономическими вызовами, и в такие моменты важно иметь доступ к дополнительным средствам. На сайте [url=https://dengizaimy.ru/]деньги в долг Минск[/url] вам помогут с минимальными усилиями. В качестве официального представителя Брокерс Групп, я с удовольствием делиться этой важной информацией, чтобы помочь людям, кто нуждается в финансовой поддержке. Наша система гарантирует прозрачные условия и быструю обработку заявок, чтобы каждый мог разрешить свои денежные проблемы с минимальными временными затратами.
http://womangu.ru
В наше время многие из нас сталкиваются с внезапными затратами и денежными трудностями, и в такие моменты важно иметь доступ к дополнительным средствам. На сайте [url=https://dengizaimy.ru/]ДеньгиЗаймы[/url] вам помогут быстро и удобно. В качестве официального представителя Брокерс Групп, я с удовольствием делиться этой информацией, чтобы помочь каждому, кто столкнулся с финансовыми трудностями. Наша платформа гарантирует честные условия и быструю обработку заявок, чтобы каждый мог решить свои финансовые вопросы быстро и эффективно.
В наше время многие из нас сталкиваются с непредвиденными расходами и экономическими вызовами, и в такие моменты важно иметь доступ к дополнительным средствам. На сайте [url=https://dengizaimy.ru/]займы онлайн на карту[/url] вам помогут быстро и удобно. В качестве официального представителя Брокерс Групп, я рад делиться этой важной информацией, чтобы помочь людям, кто нуждается в финансовой поддержке. Наша система гарантирует честные условия и быструю обработку заявок, чтобы каждый мог решить свои финансовые вопросы быстро и эффективно.
В наше время многие из нас сталкиваются с внезапными затратами и экономическими вызовами, и в такие моменты важно иметь возможность взять кредит. На сайте [url=https://dengizaimy.ru/]деньги в долг срочно[/url] вам помогут с минимальными усилиями. В качестве официального представителя Брокерс Групп, я с удовольствием делиться этой информацией, чтобы помочь каждому, кто нуждается в финансовой поддержке. Наша система гарантирует прозрачные условия и быструю обработку заявок, чтобы каждый мог решить свои финансовые вопросы с минимальными временными затратами.
[url=https://azithromycinmds.com/]azithromycin tablets over the counter[/url]
В наше время многие из нас сталкиваются с внезапными затратами и денежными трудностями, и в такие моменты важно иметь доступ к дополнительным средствам. На сайте [url=https://dengizaimy.ru/]деньги в долг в Минске[/url] вам помогут с минимальными усилиями. В качестве официального представителя Брокерс Групп, я рад делиться этой информацией, чтобы помочь людям, кто столкнулся с финансовыми трудностями. Наша платформа гарантирует честные условия и быструю обработку заявок, чтобы каждый мог разрешить свои денежные проблемы быстро и эффективно.
В наше время многие из нас сталкиваются с непредвиденными расходами и экономическими вызовами, и в такие моменты важно иметь возможность взять кредит. На сайте [url=https://dengizaimy.ru/]оформить займ[/url] вам помогут быстро и удобно. В качестве официального представителя Брокерс Групп, я с удовольствием делиться этой информацией, чтобы помочь людям, кто столкнулся с финансовыми трудностями. Наша платформа гарантирует честные условия и быструю обработку заявок, чтобы каждый мог разрешить свои денежные проблемы с минимальными временными затратами.
Pingback: wikipedia acarbose
В наше время многие из нас сталкиваются с внезапными затратами и экономическими вызовами, и в такие моменты важно иметь доступ к дополнительным средствам. На сайте [url=https://dengizaimy.ru/]займ денег[/url] вам помогут быстро и удобно. В качестве официального представителя Брокерс Групп, я с удовольствием делиться этой информацией, чтобы помочь людям, кто столкнулся с финансовыми трудностями. Наша платформа гарантирует прозрачные условия и оперативное рассмотрение запросов, чтобы каждый мог решить свои финансовые вопросы с минимальными временными затратами.
В наше время многие из нас сталкиваются с внезапными затратами и экономическими вызовами, и в такие моменты важно иметь доступ к дополнительным средствам. На сайте [url=https://dengizaimy.ru/]займ в Минске[/url] вам помогут быстро и удобно. В качестве официального представителя Брокерс Групп, я с удовольствием делиться этой важной информацией, чтобы помочь людям, кто столкнулся с финансовыми трудностями. Наша платформа гарантирует честные условия и оперативное рассмотрение запросов, чтобы каждый мог разрешить свои денежные проблемы с минимальными временными затратами.
На сайте https://start.shkolnayayarmarka.ru/ воспользуйтесь выгодными предложениями, связанными с приобретением товаров за образовательную валюту. Также вы сможете опубликовать свою продукцию на благотворительной ярмарке. Получить умникоины получится без скачивания и регистрации. Изучите все бренды, которые разместили свои товары. А в обмен ученики должны подтвердить свой уровень знаний. Воспользоваться предложением могут и талантливые студенты. Разместите свои бенефиты прямо сейчас.
Pingback: abilify and weed
Услуга по сносу старых домов и вывозу мусора в Москве и Московской области. Мы предоставляем услуги по сносу старых зданий и удалению мусора на территории Москвы и Подмосковья. Услуга http://demontazh-doma-msk5.ru выполняется опытными специалистами в течение 24 часов после оформления заказа. Перед началом работ наш эксперт бесплатно приезжает на объект для оценки объёма работ и консультации. Чтобы получить дополнительную информацию и рассчитать стоимость услуг, свяжитесь с нами по телефону или оставьте заявку на сайте компании.
[url=https://tadalafilstd.com/]tadalafil 20mg cheap[/url]
В сегодняшнем обществе финансовые сложности встречаются часто, и каждый человек может оказаться в ситуации, когда потребуется дополнительная финансовая помощь. На сайте [url=https://moneybel.ru/]займ в беларуси[/url] помогут решить эти проблемы, принося простой и удобный способ получения кредита. Мы понимаем, что время играет ключевую роль, поэтому наш процесс обработки заявок максимально быстр и эффективен. В роли представителя Манибел, наша цель – помочь каждому клиенту преодолеть финансовые затруднения, предложив ясные и выгодные условия займа. Доверьтесь нашей компании, и мы поможем вам в решении ваших финансовых проблем.
В сегодняшнем обществе финансовые трудности встречаются часто, и каждый из нас может оказаться в ситуации, когда потребуется дополнительная финансовая помощь. На сайте [url=https://moneybel.ru/]займ денег[/url] помогут преодолеть эти трудности, принося простой и лёгкий метод займа. Мы осознаём, что время играет ключевую роль, поэтому наш процесс обработки заявок максимально быстр и эффективен. В роли представителя Манибел, мы нацелены на то, чтобы помочь каждому клиенту преодолеть свои денежные трудности, предоставив прозрачные и выгодные условия кредитования. Доверьтесь нашей компании, и мы решим ваши финансовые проблемы.
В настоящей эпохе финансовые трудности встречаются часто, и каждый человек может оказаться в ситуации, когда потребуется дополнительная финансовая помощь. На сайте [url=https://moneybel.ru/]срочно взять деньги[/url] помогут решить эти проблемы, принося простой и лёгкий метод займа. Мы понимаем, что время играет ключевую роль, поэтому наш процесс обработки заявок максимально быстр и эффективен. В качестве представителя Манибел, наша цель – помочь каждому клиенту преодолеть финансовые затруднения, предложив ясные и выгодные условия займа. Доверьтесь нам, и мы решим ваши финансовые проблемы.
В сегодняшнем обществе финансовые сложности не редкость, и каждый человек может оказаться в ситуации, когда необходима дополнительная финансовая поддержка. На сайте [url=https://moneybel.ru/]займы онлайн на карту[/url] помогут преодолеть эти трудности, принося простой и лёгкий метод займа. Мы понимаем, что времени на решение проблемы не хватает, поэтому наш процесс обработки заявок максимально быстр и эффективен. В роли представителя Манибел, наша цель – помочь каждому клиенту преодолеть финансовые затруднения, предоставив прозрачные и выгодные условия кредитования. Доверьтесь нашей компании, и мы поможем вам в решении ваших финансовых проблем.
В сегодняшнем обществе финансовые сложности не редкость, и каждый из нас может оказаться в ситуации, когда потребуется дополнительная финансовая помощь. На сайте [url=https://moneybel.ru/]займ онлайн[/url] помогут решить эти проблемы, принося простой и удобный способ получения кредита. Мы осознаём, что время играет ключевую роль, поэтому наш процесс обработки заявок максимально быстр и эффективен. В роли представителя Манибел, мы нацелены на то, чтобы помочь каждому клиенту преодолеть свои денежные трудности, предоставив прозрачные и выгодные условия кредитования. Доверьтесь нашей компании, и мы решим ваши финансовые проблемы.
Stumbled upon a unique article, I suggest you take a look https://www.redcuprebellion.com/users/cchatruletka
Pingback: actos ecologicos
В сегодняшнем обществе финансовые трудности не редкость, и каждый человек может оказаться в ситуации, когда необходима дополнительная финансовая поддержка. На сайте [url=https://moneybel.ru/]moneybel.ru[/url] помогут решить эти проблемы, предоставляя простой и удобный способ займа. Мы понимаем, что время играет ключевую роль, поэтому мы обрабатываем заявки оперативно и эффективно. В качестве представителя Манибел, мы нацелены на то, чтобы помочь каждому клиенту преодолеть свои денежные трудности, предоставив прозрачные и выгодные условия кредитования. Доверьтесь нам, и мы решим ваши финансовые проблемы.
На сайте https://grsuv.ru/ закажите такую полезную и нужную услугу, как гравировка, которая выполняется на жетонах, ложках, а также шильдах. Гравировка может быть выполнена на самых разных вещах. В компании также производят и клише, выполняют гравировку по кругу. При необходимости специалист приедет непосредственно к заказчику, чтобы выполнить все необходимые работы. Все услуги оказываются на инновационном оборудовании, а потому только высокого качества. Специально для вас специалист подберет шрифты и выполнит любую надпись. Будут написаны имена на любых столовых приборах, выполненных из серебра либо нержавеющей стали.
В настоящей эпохе финансовые сложности не редкость, и каждый из нас может оказаться в ситуации, когда потребуется дополнительная финансовая помощь. На сайте [url=https://moneybel.ru/]получить деньги[/url] помогут преодолеть эти трудности, принося простой и лёгкий метод займа. Мы осознаём, что времени на решение проблемы не хватает, поэтому наш процесс обработки заявок максимально быстр и эффективен. В качестве представителя Манибел, мы нацелены на то, чтобы помочь каждому клиенту преодолеть свои денежные трудности, предложив ясные и выгодные условия займа. Доверьтесь нам, и мы решим ваши финансовые проблемы.
Зайдите на сайт https://easypayments.online/payments и вы сможете открыть компанию за границей, получить аккаунты в платежных системах и начать принимать платежи на сайте со всего мира. Регистрация Stripe и Paypal для приема платежей со всего мира от Easy Payments это надежный способ легально принимать платежи.
Привет всем!
Современные смартфоны – это не просто устройства для связи, это настоящие мультимедийные центры и помощники в повседневной жизни. Мы расскажем вам о всех новых функциях и возможностях, которые предлагают ведущие бренды мобильных устройств.
Наша команда экспертов следит за всеми главными новостями и разработками в мире смартфонов, чтобы вы всегда были в курсе самых актуальных событий. Мы предлагаем вам качественные материалы и аналитику о самых важных аспектах мобильной технологии.
Все самое лучшее на сайте https://formobile.top/category/game/
[url=https://formobile.top/category/smartphone-news/]смартфоны новости[/url]
hi-tech новости технологий
новости про смартфоны
смартфоны новости
Удачи!
Хотите сделать заказ решеток на окна? Обращайтесь в компанию «РУСРЕШЕТКА». Мы хорошо знаем свою работу и делаем ее с любовью. Предложим вам оптимальное решение. Принимаем к оплате наличные и безналичные переводы. Ищете кованная перила? Rusreshetka.ru – сайт, где представлен каталог товаров, ознакомиться с ним можете в любое удобное время. Гарантируем быструю доставку и заманчивую цену. Позвоните нам и закажите решетки на окна с установкой. Мы всегда готовы ответить на интересующие вопросы, помочь сделать правильный выбор и принять заявку.
В настоящей экономической обстановке, где неожиданные финансовые обязательства могут возникнуть в любое время, поиск надежного и удобного источника финансовой помощи становится все более критическим. Предложение [url=https://zaim-minsk.ru/]деньги в долг в Минске срочно[/url] предлагает возможность рассчитывать на быстрое и беззаботное получение необходимых средств без лишних трудностей. Мы ценим ваше время и обеспечиваем оперативную обработку всех запросов. В качестве вашего финансового партнера, наша главная задача состоит в том, чтобы помочь вам преодолеть текущие финансовые препятствия, предоставляя ясные и выгодные условия кредитования. Доверьтесь нам в вашем финансовом путешествии, и мы обеспечим вас надежной поддержкой на каждом этапе.
В настоящей экономической обстановке, где неожиданные финансовые обязательства могут возникнуть в любое время, поиск надежного и удобного источника финансовой помощи становится все более критическим. Предложение [url=https://zaim-minsk.ru/]взять деньги[/url] предлагает возможность рассчитывать на быстрое и беззаботное получение необходимых средств без лишних трудностей. Мы ценим ваше время и обеспечиваем оперативную обработку всех запросов. В качестве вашего финансового партнера, наша главная задача состоит в том, чтобы помочь вам преодолеть текущие финансовые препятствия, предоставляя ясные и выгодные условия кредитования. Доверьтесь нам в вашем финансовом путешествии, и мы обеспечим вас надежной поддержкой на каждом этапе.
В настоящей экономической обстановке, где неожиданные финансовые обязательства могут возникнуть в любое время, поиск надежного и удобного источника финансовой помощи становится все более критическим. Предложение [url=https://zaim-minsk.ru/]взять деньги в долг в Минске[/url] предлагает возможность рассчитывать на быстрое и беззаботное получение необходимых средств без лишних трудностей. Мы ценим ваше время и обеспечиваем оперативную обработку всех запросов. В качестве вашего финансового партнера, наша главная задача состоит в том, чтобы помочь вам преодолеть текущие финансовые препятствия, предоставляя ясные и выгодные условия кредитования. Доверьтесь нам в вашем финансовом путешествии, и мы обеспечим вас надежной поддержкой на каждом этапе.
В настоящей экономической обстановке, где неожиданные финансовые обязательства могут возникнуть в любое время, поиск надежного и удобного источника финансовой помощи становится все более критическим. Предложение [url=https://zaim-minsk.ru/]взять денег в долг срочно[/url] предлагает возможность рассчитывать на быстрое и беззаботное получение необходимых средств без лишних трудностей. Мы ценим ваше время и обеспечиваем оперативную обработку всех запросов. В качестве вашего финансового партнера, наша главная задача состоит в том, чтобы помочь вам преодолеть текущие финансовые препятствия, предоставляя ясные и выгодные условия кредитования. Доверьтесь нам в вашем финансовом путешествии, и мы обеспечим вас надежной поддержкой на каждом этапе.
В настоящей экономической обстановке, где неожиданные финансовые обязательства могут возникнуть в любое время, поиск надежного и удобного источника финансовой помощи становится все более критическим. Предложение [url=https://zaim-minsk.ru/]взять деньги в долг в Минске[/url] предлагает возможность рассчитывать на быстрое и беззаботное получение необходимых средств без лишних трудностей. Мы ценим ваше время и обеспечиваем оперативную обработку всех запросов. В качестве вашего финансового партнера, наша главная задача состоит в том, чтобы помочь вам преодолеть текущие финансовые препятствия, предоставляя ясные и выгодные условия кредитования. Доверьтесь нам в вашем финансовом путешествии, и мы обеспечим вас надежной поддержкой на каждом этапе.
В настоящей экономической обстановке, где неожиданные финансовые обязательства могут возникнуть в любое время, поиск надежного и удобного источника финансовой помощи становится все более критическим. Предложение [url=https://zaim-minsk.ru/]займы онлайн[/url] предлагает возможность рассчитывать на быстрое и беззаботное получение необходимых средств без лишних трудностей. Мы ценим ваше время и обеспечиваем оперативную обработку всех запросов. В качестве вашего финансового партнера, наша главная задача состоит в том, чтобы помочь вам преодолеть текущие финансовые препятствия, предоставляя ясные и выгодные условия кредитования. Доверьтесь нам в вашем финансовом путешествии, и мы обеспечим вас надежной поддержкой на каждом этапе.
Pingback: protonix vs nexium
В настоящей экономической обстановке, где неожиданные финансовые обязательства могут возникнуть в любое время, поиск надежного и удобного источника финансовой помощи становится все более критическим. Предложение [url=https://zaim-minsk.ru/]деньги в долг[/url] предлагает возможность рассчитывать на быстрое и беззаботное получение необходимых средств без лишних трудностей. Мы ценим ваше время и обеспечиваем оперативную обработку всех запросов. В качестве вашего финансового партнера, наша главная задача состоит в том, чтобы помочь вам преодолеть текущие финансовые препятствия, предоставляя ясные и выгодные условия кредитования. Доверьтесь нам в вашем финансовом путешествии, и мы обеспечим вас надежной поддержкой на каждом этапе.
На сайте https://prokat.car-trip.ru/ вы можете взять в аренду автомобили среднего и экономичного класса по привлекательной стоимости. Компания имеет большой опыт, позволяющий предлагать ей исключительно качественные услуги. Здесь вы найдете больше 100 импортных автомобилей на любой вкус и для разных задач. Доставка осуществляется в любую точку города. Все авто застрахованы. Всегда готовы вам помочь в выборе и ответить на все ваши интересующие вопросы. Подробнее ознакомиться с услугами, а также условиями аренды вы можете на сайте уже сейчас.
Услуга по сносу старых домов и утилизации мусора в Москве и Московской области. Мы предлагаем услуги по сносу старых построек и удалению отходов на территории Москвы и Московской области. Услуга разборка сруба предоставляется опытными специалистами в течение 24 часов после оформления заказа. Перед началом работ наш эксперт бесплатно посещает объект для определения объёма работ и предоставления консультаций. Чтобы получить дополнительную информацию и рассчитать стоимость услуг, свяжитесь с нами по телефону или оставьте заявку на веб-сайте компании.
Внимание, любители ставок! БК Олимп представляет с гордостью последнюю версию своего приложения для Android, которое обещает изменить ваш мир ставок на радость. Мы обновили приложение, чтобы гарантировать лучший впечатление от ставок на спорт. [url=https://top-olimp-apk.ru/]Скачать сайт Олимп[/url] и откройте доступ к широкому спектру спортивных событий, который стал удобнее благодаря новому дизайну и ускоренной обработке данных. Подключайтесь к нам и наслаждайтесь возможностью делать ставки в любой точке мира, будь то дома или в отпуске. Получите новую версию приложения прямо сейчас и погрузитесь в мир ставок с БК Олимп, где победы становятся реальностью!
Внимание, любители ставок! БК Олимп представляет с гордостью новейшую версию своего приложения для Android, которое обещает перевернуть ваш мир ставок на радость. Мы полностью пересмотрели приложение, чтобы гарантировать несравненный опыт от ставок на спорт. [url=https://top-olimp-apk.ru/]Приложение Olimp[/url] и откройте просмотр разнообразных спортивных событий, который стал удобнее благодаря улучшенному интерфейсу и ускоренной обработке данных. Присоединяйтесь к сообществу БК Олимп и пользуйтесь возможностью делать ставки в любой точке мира, будь то на диване или в отпуске. Загрузите это обновление приложения прямо сейчас и откройте для себя мир ставок с БК Олимп, где выигрыши становятся реальностью!
На сайте https://smartflow.ru ознакомьтесь с сантехникой премиального уровня. Вся продукция создана из инновационных, высокотехнологичных материалов и с использованием уникальных, новаторских технологий. Продукты разработаны тщательным образом, чтобы предложить вам лучшее. Сантехника гарантирует безупречный уровень функциональности, эстетичности. Она наделена долгим сроком эксплуатации. Идеально впишется в концепцию. Представлены «умные» модели унитазов безупречного качества.
На сайте http://weller.ru/stroitelstvo/vinilovyj-sajding-obzor-oblicovochnogo-materiala/ ознакомьтесь с информацией, посвященной виниловому сайдингу. Имеется вся важная информация, которая касается характеристик, внешнего вида, особенностей такого материала. Опубликована и история появления винилового сайдинга, и другая ценная информация. Вместе с тем, вы узнаете о достоинствах материала, почему его выбирает большинство. Кроме того, перечислены и современные производители. Узнаете, где можно приобрести такой строительный материал по бюджетной стоимости.
На сайте https://iz-zala.ru/ находится каталог товаров. Здесь каждый желающий получает возможность составить описание продукции, опубликовать самые популярные товары, а также актуальные акции. В специальном поле разместите побольше данных для того, чтобы вашим предложением заинтересовались. Текст в блоках можно форматировать, выделять различные его части, а также особенно важные элементы, добавлять картинки и делать все, что считаете нужным. Также можно ссылаться и на другие документы. Необходимо рассказать о компании очень подробно.
Внимание, любители ставок! БК Олимп представляет с гордостью новейшую версию своего приложения для Android, которое обещает перевернуть ваш мир ставок на радость. Мы обновили приложение, чтобы гарантировать лучший впечатление от ставок на спорт. [url=https://top-olimp-apk.ru/]Букмекерская контора Олимп на андроид[/url] и откройте доступ к широкому спектру спортивных событий, который стал удобнее благодаря новому дизайну и ускоренной обработке данных. Присоединяйтесь к сообществу БК Олимп и пользуйтесь возможностью делать ставки в любой точке мира, будь то на диване или в путешествии. Загрузите это обновление приложения без задержек и погрузитесь в мир ставок с БК Олимп, где выигрыши становятся реальностью!
Внимание, любители ставок! БК Олимп представляет с гордостью последнюю версию своего приложения для Android, которое обещает изменить ваш мир ставок к лучшему. Мы полностью пересмотрели приложение, чтобы предоставить вам лучший опыт от ставок на спорт. [url=https://top-olimp-apk.ru/]Скачать приложение конторы Олимп[/url] и откройте просмотр разнообразных спортивных событий, который стал удобнее благодаря новому дизайну и оптимизированной производительности данных. Присоединяйтесь к сообществу БК Олимп и пользуйтесь возможностью делать ставки где угодно, будь то на диване или в отпуске. Получите это обновление приложения прямо сейчас и откройте для себя мир ставок с БК Олимп, где победы становятся реальностью!
Внимание, любители ставок! БК Олимп представляет с гордостью новейшую версию своего приложения для Android, которое обещает изменить ваш мир ставок к лучшему. Мы обновили приложение, чтобы предоставить вам несравненный впечатление от ставок на спорт. [url=https://top-olimp-apk.ru/]Приложение Olimp[/url] и откройте просмотр разнообразных спортивных событий, который стал удобнее благодаря улучшенному интерфейсу и ускоренной обработке данных. Присоединяйтесь к сообществу БК Олимп и пользуйтесь возможностью делать ставки где угодно, будь то на диване или в путешествии. Получите новую версию приложения прямо сейчас и откройте для себя мир ставок с БК Олимп, где выигрыши становятся реальностью!
[url=https://happyfamilymedicalstore.online/]best online pharmacy reddit[/url]
кондиционеры сплит системы [url=https://multisplit-sistemy-kondicionirovaniya.ru/]кондиционеры сплит системы[/url] .
Вот оно, свежее обновление от БК Олимп для всех энтузиастов ставок с Android устройствами! Преобразив наше приложение, мы стремимся предоставить вам уникальный опыт, делая ставки не только удобными, но и захватывающими. [url=https://best-olimpbet-app.ru/]Олимп бет на андроид[/url] доступное для игроков! С улучшенным интерфейсом и ускоренной производительностью, доступ к большому разнообразию событий стал легким делом. Испытайте радость от улучшенного управления счетом и простоты использования, которые делают каждую ставку особым событием. Не упустите возможностях – установите последнее обновление немедленно и вступите в ряды довольных пользователей, празднующих каждой ставке с БК Олимп, где каждый шанс на победу высоко оценивается.
Вот оно, свежее веяние от БК Олимп для всех энтузиастов ставок с Android устройствами! Преобразив наше приложение, мы целимся предоставить вам уникальный опыт, делая ставки не только удобными, но и захватывающими. [url=https://best-olimpbet-app.ru/]Приложение Olimp на андроид[/url] доступно для игроков! С новым дизайном и быстрой обработкой данных, доступ к большому разнообразию событий стал проще простого. Испытайте радость от оптимизированной работы с аккаунтом и интуитивной навигации, которые делают каждую ставку особым событием. Не упустите возможностях – установите последнее обновление сейчас же и присоединитесь к довольных пользователей, празднующих каждой ставке с БК Олимп, где каждый шанс на победу высоко оценивается.
Вот оно, свежее обновление от БК Олимп для всех энтузиастов ставок с Android устройствами! Изменив наше приложение, мы стремимся предоставить вам непревзойденный опыт, делая ставки не только удобными, но и захватывающими. [url=https://best-olimpbet-app.ru/]Бесплатно скачать Олимп бет на андроид[/url] возможно сегодня! С новым дизайном и быстрой обработкой данных, доступ к огромному спектру событий стал проще простого. Испытайте радость от оптимизированной работы с аккаунтом и простоты использования, которые делают каждую ставку особым событием. Забудьте о пропущенных возможностях – скачайте последнее обновление сейчас же и вступите в ряды довольных пользователей, празднующих каждой ставке с БК Олимп, где каждый шанс на победу ценится.
Вот оно, свежее веяние от БК Олимп для всех поклонников ставок с Android устройствами! Изменив наше приложение, мы целимся предоставить вам непревзойденный опыт, делая ставки не только простыми, но и захватывающими. [url=https://best-olimpbet-app.ru/]Приложение Олимп бет на андроид[/url] доступно сейчас! С улучшенным интерфейсом и быстрой обработкой данных, доступ к огромному спектру событий стал легким делом. Ощутите радость от улучшенного управления счетом и интуитивной навигации, которые делают каждую ставку особым событием. Забудьте о пропущенных возможностях – скачайте последнее обновление сейчас же и вступите в ряды довольных пользователей, празднующих каждой ставке с БК Олимп, где каждый шанс на победу ценится.
Вот оно, свежее веяние от БК Олимп для всех энтузиастов ставок с Android устройствами! Изменив наше приложение, мы стремимся предоставить вам уникальный опыт, делая ставки не только удобными, но и захватывающими. [url=https://best-olimpbet-app.ru/]Скачать сайт Олимп[/url] возможно сегодня! С улучшенным интерфейсом и быстрой обработкой данных, доступ к огромному спектру событий стал проще простого. Испытайте радость от оптимизированной работы с аккаунтом и простоты использования, которые делают каждую ставку волнующим моментом. Забудьте о пропущенных возможностях – скачайте последнее обновление сейчас же и вступите в ряды довольных пользователей, празднующих каждой ставке с БК Олимп, где каждый шанс на победу высоко оценивается.
Вот оно, свежее обновление от БК Олимп для всех поклонников ставок с Android устройствами! Изменив наше приложение, мы целимся предоставить вам непревзойденный опыт, делая ставки не только удобными, но и захватывающими. [url=https://best-olimpbet-app.ru/]Скачать Olimp bet[/url] уже сегодня! С новым дизайном и быстрой обработкой данных, доступ к огромному спектру событий стал проще простого. Испытайте радость от оптимизированной работы с аккаунтом и интуитивной навигации, которые делают каждую ставку особым событием. Забудьте о пропущенных возможностях – установите последнее обновление сейчас же и вступите в ряды довольных пользователей, празднующих каждой ставке с БК Олимп, где каждый шанс на победу ценится.
Не пропустите обновление от БК Олимп для Android! [url=https://skachat-olimp-apk.ru/]БК Олимп для андроид[/url] доступно для всех. Мы пересмотрели наше приложение, чтобы ваш опыт ставок стал ещё лучше. С переработанным дизайном и повышенной скоростью, вы получите доступ к широкому ассортименту спортивных событий легко и просто. Наслаждайтесь удобством ведения счета и лёгкостью использования, делая каждую ставку особым моментом. Загрузите это приложение прямо сейчас и начните выигрывать с БК Олимп, где каждая ставка приносит удовольствие!
Не пропустите новинку от БК Олимп для Android! [url=https://skachat-olimp-apk.ru/]Скачать apk Olimp bet[/url] возможно сегодня. Мы обновили наше приложение, чтобы ваш опыт ставок стал ещё лучше. С переработанным дизайном и ускоренной обработкой данных, вы получите доступ к широкому ассортименту спортивных событий легко и просто. Наслаждайтесь простотой управления аккаунтом и лёгкостью использования, делая каждую ставку особым моментом. Загрузите это приложение прямо сейчас и стартуйте побеждать с БК Олимп, где каждая ставка приносит радость!
Не пропустите новинку от БК Олимп для Android! [url=https://skachat-olimp-apk.ru/]Приложение Olimp[/url] уже сегодня. Мы пересмотрели наше приложение, чтобы ваш опыт ставок стал ещё удобнее. С новым интерфейсом и повышенной скоростью, вы получите доступ к многообразию спортивных событий легко и просто. Наслаждайтесь простотой управления аккаунтом и интуитивной навигацией, делая каждую ставку значимым событием. Установите это обновление прямо сейчас и начните побеждать с БК Олимп, где каждая ставка приносит радость!
На сайте https://ack-group.ru/ воспользуйтесь услугами психологического центра, в котором работают высококлассные, опытные специалисты с огромным стажем. Они никогда не критикуют, а подталкивают к тому, чтобы вы сами осознали проблему и приняли правильное решение. Очень часто устраиваются акции, чтобы вы сэкономили семейный бюджет. Психологи помогают пациентам справиться с любой проблемой, независимо от сложности. Основная специализация – это финансы, личные отношения. Если и вы хотите изменений в личной жизни и хотите улучшить свое положение, то воспользуйтесь услугами специалистов.
Услуга по сносу старых зданий и утилизации отходов в Москве и Московской области. Мы предоставляем услуги по сносу старых сооружений и удалению мусора на территории Москвы и Московской области. Услуга разобрать дом выполняется квалифицированными специалистами в течение 24 часов после оформления заказа. Перед началом работ наш эксперт бесплатно посещает объект для определения объёма работ и предоставления консультаций. Чтобы получить дополнительную информацию и рассчитать стоимость услуг, свяжитесь с нами по телефону или оставьте заявку на сайте компании.
Не пропустите обновление от БК Олимп для Android! [url=https://skachat-olimp-apk.ru/]Приложение БК Олимп[/url] доступно для всех. Мы пересмотрели наше приложение, чтобы ваш опыт ставок стал ещё лучше. С переработанным дизайном и ускоренной обработкой данных, вы получите доступ к широкому ассортименту спортивных событий без усилий. Наслаждайтесь удобством ведения счета и интуитивной навигацией, делая каждую ставку значимым событием. Загрузите это приложение сегодня и начните выигрывать с БК Олимп, где каждая ставка приносит радость!
Не пропустите новинку от БК Олимп для Android! [url=https://skachat-olimp-apk.ru/]Скачать БК Олимп на андроид[/url] уже сегодня. Мы пересмотрели наше приложение, чтобы ваш опыт ставок стал ещё лучше. С переработанным дизайном и повышенной скоростью, вы получите доступ к многообразию спортивных событий без усилий. Наслаждайтесь простотой управления аккаунтом и лёгкостью использования, делая каждую ставку особым моментом. Установите это обновление сегодня и стартуйте выигрывать с БК Олимп, где каждая ставка приносит радость!
Не пропустите обновление от БК Олимп для Android! [url=https://skachat-olimp-apk.ru/]Скачать Олимп бесплатно[/url] доступно для всех. Мы обновили наше приложение, чтобы ваш опыт ставок стал ещё удобнее. С переработанным дизайном и повышенной скоростью, вы получите доступ к широкому ассортименту спортивных событий легко и просто. Оцените удобством ведения счета и интуитивной навигацией, делая каждую ставку особым моментом. Установите это обновление сегодня и стартуйте выигрывать с БК Олимп, где каждая ставка приносит удовольствие!
https://kursovuyupishem.ru/
Выбирайте на ресурсе грифы для штанг Grify-Dlya-Shtang с разным посадочным диаметром для силовых тренировок. Это востребованное спортивное оборудование для отдела свободных весов в фитнес клубах.Российский изготовитель предлагает большой ассортимент слабоизогнутых W грифов по недорогим ценам напрямую у завода-производителя. Все наборы поступают в продажу с фиксаторами для блинов. Российский завод производит инвентарь из стали лучших марок. Выпускаемые изделия не нуждаются в постоянном обслуживании и ориентированы на длительную эксплуатацию в спортивных залах. Для защиты от ударов все металлические поверхности хромируются. Приобретая у отечественного изготовителя вы получаете эффективные снаряды для безопасных тренировок.
Ищите карточный домик смотреть онлайн? Зайдите на сайт https://kino-domik.ru/ и вы найдете все сезоны карточный домик в отличном качестве. Если вы не смотрели сериал обязательно посмотрите. Политическая драма захватывает дух, а каждая серия преподносит что то новое и интересное.
На сайте https://akvapark-fentazi.ru/ изучите важную и полезную информацию об аквапарке «Фэнтези». Вы находитесь непосредственно на официальном сайте. Это подходящее место, в котором сможет полноценно отдохнуть вся семья, а особенно дети. На стоянке вы сможете оставить автомобиль и насладиться временем, проведенным с семьей. При этом стоимость билета остается на доступном уровне. Каждый посетитель сможет воспользоваться СПА-зоной, поплавать в бассейне, полакомиться вкусными блюдами.
Запуск переделанной версии приложения БК Олимп для Android уже здесь! Модернизировав функционал, мы сделали процесс ставок еще более увлекательным. [url=https://skachat-olimpbet.ru/]Олимп бет на андроид[/url] доступно для ставочников. С обновленным дизайном и ускоренной загрузкой данных, доступ к разнообразию спортивных событий станет игрой. Наслаждайтесь простотой управления счетом и интуитивно понятной навигацией, превращая каждую ставку в особенный момент. Не теряйте времени, скачайте последнюю версию без отлагательств и присоединяйтесь к победителям с БК Олимп, где ставки приносят удовольствие и победы!
Запуск обновленной версии приложения БК Олимп для Android уже здесь! Переосмыслив функционал, мы сделали процесс ставок невероятно простым. [url=https://skachat-olimpbet.ru/]Скачать приложение Олимп бет[/url] доступное для игроков. С передовым интерфейсом и ускоренной загрузкой данных, доступ к разнообразию спортивных событий станет легкостью. Ощутите удобство управления счетом и легкой навигацией, превращая каждую ставку в особенный момент. Не теряйте времени, скачайте последнюю версию прямо сейчас и присоединяйтесь к удовлетворенным пользователям с БК Олимп, где ставки приносят удовольствие и победы!
Evakmoscow https://evakmoscow.ru/ это Служба эвакуации автомобилей в Москве и области с подачей от 15 минут. Имеем собственный автопарк. Помогаем с эвакуацией мотоциклов, легковых автомобилей, внедорожников. Эвакуируем коммерческий, грузовой транспорт и спецтехнику. Подробнее на сайте.
Запуск переделанной версии приложения БК Олимп для Android уже здесь! Модернизировав функционал, мы сделали процесс ставок невероятно простым. [url=https://skachat-olimpbet.ru/]БК Олимп для андроид[/url] доступно для ставочников. С обновленным дизайном и быстрой обработкой, доступ к всему спектру спортивных событий станет легкостью. Ощутите удобство управления счетом и интуитивно понятной навигацией, превращая каждую ставку в важное событие. Не задерживайтесь, скачайте последнюю версию без отлагательств и присоединяйтесь к удовлетворенным пользователям с БК Олимп, где ставки приносят наслаждение и победы!
Запуск переделанной версии приложения БК Олимп для Android уже здесь! Переосмыслив функционал, мы сделали процесс ставок невероятно простым. [url=https://skachat-olimpbet.ru/]Бесплатно скачать Олимп бет на андроид[/url] доступное для игроков. С передовым интерфейсом и быстрой обработкой, доступ к разнообразию спортивных событий станет игрой. Ощутите удобство управления счетом и интуитивно понятной навигацией, превращая каждую ставку в особенный момент. Не теряйте времени, скачайте последнюю версию прямо сейчас и присоединяйтесь к победителям с БК Олимп, где ставки приносят удовольствие и победы!
Запуск обновленной версии приложения БК Олимп для Android уже здесь! Переосмыслив функционал, мы сделали процесс ставок еще более увлекательным. [url=https://skachat-olimpbet.ru/]БК Олимп для андроид[/url] доступно для ставочников. С передовым интерфейсом и быстрой обработкой, доступ к всему спектру спортивных событий станет легкостью. Ощутите удобство управления счетом и легкой навигацией, превращая каждую ставку в важное событие. Не теряйте времени, скачайте последнюю версию прямо сейчас и присоединяйтесь к победителям с БК Олимп, где ставки приносят наслаждение и победы!
Байкальские легенды – компания, которая сделает отдых незабываемым. Наши туристы являются главным для нас. Экскурсии все запомнятся надолго, они очень интересные. Ищете экскурсионные туры на Байкал? Baikallegends.com – сайт, где представлены многочисленные отзывы довольных клиентов. Здесь также есть фото с туров, посмотрите их уже сейчас. Мы относимся с любовью к своему делу, гарантируем профессиональное и качественное обслуживание. При возникновении вопросов, обращайтесь к нам. Невероятные красоты Байкала ждут вас!
Откройте для себя последние новшества в приложении БК Олимп для Android! [url=https://olimp-apk.ru/]Бесплатно скачать Олимп бет на андроид[/url] уже сегодня. Мы улучшили наше приложение, чтобы сделать ваше взаимодействие с ставками удобным. Благодаря усовершенствованному интерфейсу и быстрой загрузке данных, вы теперь имеете прямой доступ к широкому ассортименту спортивных событий. Научитесь наслаждаться удобство использования своим аккаунтом и интуитивную навигацию, превращая каждую вашу ставку в уникальное приключение. Получите приложение сейчас и начните получать удовольствие от побед вместе с БК Олимп, где каждая ставка несет радость.
Came across an intriguing article – it’s worth your attention, trust me http://altla2.mybb.ru/viewtopic.php?id=428#p636
Откройте для себя последние новшества в приложении БК Олимп для Android! [url=https://olimp-apk.ru/]Скачать приложение БК Олимп[/url] доступное для игроков. Мы совершенствовали наше приложение, чтобы сделать ваше взаимодействие с ставками более приятным. Благодаря усовершенствованному интерфейсу и ускоренной обработке данных, вы теперь имеете прямой доступ к широкому ассортименту спортивных событий. Оцените удобство использования своим аккаунтом и легкость управления, превращая каждую вашу ставку в уникальное приключение. Загрузите обновление прямо сегодня и начните получать удовольствие от побед вместе с БК Олимп, где каждая ставка принесет успех.
Откройте для себя последние новшества в приложении БК Олимп для Android! [url=https://olimp-apk.ru/]Бесплатно скачать Олимп[/url] доступно для всех. Мы совершенствовали наше приложение, чтобы сделать ваше взаимодействие с ставками более приятным. С новым дизайном и быстрой загрузке данных, вы теперь имеете легкий доступ к широкому ассортименту спортивных событий. Научитесь наслаждаться удобство использования своим аккаунтом и интуитивную навигацию, превращая каждую вашу ставку в значимое событие. Загрузите обновление прямо сегодня и начните получать удовольствие от побед вместе с БК Олимп, где каждая ставка несет радость.
Откройте для себя последние новшества в приложении БК Олимп для Android! [url=https://olimp-apk.ru/]Загрузить файл apk Olimp[/url] доступно сейчас. Мы совершенствовали наше приложение, чтобы сделать ваше взаимодействие с ставками более приятным. С новым дизайном и быстрой загрузке данных, вы теперь имеете легкий доступ к широкому спектру спортивных событий. Оцените простоту управления своим аккаунтом и легкость управления, превращая каждую вашу ставку в уникальное приключение. Загрузите обновление прямо сегодня и начните получать удовольствие от побед вместе с БК Олимп, где каждая ставка несет радость.
Откройте для себя последние новшества в приложении БК Олимп для Android! [url=https://olimp-apk.ru/]Приложение Олимп[/url] уже сегодня. Мы совершенствовали наше приложение, чтобы сделать ваше взаимодействие с ставками более приятным. Благодаря усовершенствованному интерфейсу и быстрой загрузке данных, вы теперь имеете прямой доступ к широкому ассортименту спортивных событий. Научитесь наслаждаться простоту управления своим аккаунтом и интуитивную навигацию, превращая каждую вашу ставку в уникальное приключение. Загрузите обновление прямо сегодня и начните зарабатывать на своих победах вместе с БК Олимп, где каждая ставка принесет успех.
Откройте для себя новейшие обновления в приложении БК Олимп для Android! [url=https://olimp-apk.ru/]Скачать Olimp bet для андроид[/url] доступно сейчас. Мы улучшили наше приложение, чтобы сделать ваше взаимодействие с ставками удобным. С новым дизайном и ускоренной обработке данных, вы теперь имеете прямой доступ к широкому ассортименту спортивных событий. Научитесь наслаждаться простоту управления своим аккаунтом и интуитивную навигацию, превращая каждую вашу ставку в уникальное приключение. Загрузите обновление сейчас и начните получать удовольствие от побед вместе с БК Олимп, где каждая ставка несет радость.
Found an enthralling read that I’d recommend – it’s truly fascinating http://uebkameri.listbb.ru/viewtopic.php?f=2&t=401
Откройте для себя новшество в мире ставок с последним обновлением приложения БК Олимп для Android! [url=https://free-apk-olimp.ru/]Olimp приложение[/url] прямо сейчас. Мы полностью переработали приложение, обеспечивая беспрецедентный уровень удобства и скорость доступа. Теперь выбор соревнований находится у вас под рукой с новым дизайном и интуитивной навигацией. Прочувствуйте на себе простоту ведения финансов и сделайте каждую ставку значительным событием. Установите это приложение немедленно и выходите на новый уровень ставок с БК Олимп, где каждый выбор может принести победу!
Откройте для себя новшество в мире ставок с новейшей версией приложения БК Олимп для Android! [url=https://free-apk-olimp.ru/]Бесплатно скачать Олимп бет на андроид[/url] прямо сейчас. Мы полностью переработали приложение, обеспечивая беспрецедентный уровень удобства и скорость доступа. Теперь разнообразие спортивных событий находится у вас под рукой с новым дизайном и легкой навигацией. Прочувствуйте на себе простоту ведения финансов и сделайте каждую ставку значительным событием. Установите это обновление сегодня и поднимайте свой опыт ставок с БК Олимп, где каждый выбор может принести победу!
Откройте для себя революцию в мире ставок с последним обновлением приложения БК Олимп для Android! [url=https://free-apk-olimp.ru/]БК Олимп на андроид[/url] сегодня и сейчас. Мы освежили приложение, обеспечивая неимоверную простоту использования и быстроту загрузки. Теперь выбор соревнований находится у вас под рукой с новым дизайном и интуитивной навигацией. Прочувствуйте на себе легкость управления счетом и сделайте каждую ставку незабываемым моментом. Установите это обновление немедленно и выходите на новый уровень ставок с БК Олимп, где каждый выбор может принести победу!
Откройте для себя новшество в мире ставок с последним обновлением приложения БК Олимп для Android! [url=https://free-apk-olimp.ru/]Скачать Олимп бет[/url] сегодня и сейчас. Мы освежили приложение, обеспечивая неимоверную простоту использования и скорость доступа. Теперь разнообразие спортивных событий находится у вас под рукой с новым дизайном и интуитивной навигацией. Прочувствуйте на себе простоту ведения финансов и сделайте каждую ставку значительным событием. Установите это обновление сегодня и поднимайте свой опыт ставок с БК Олимп, где каждый выбор может принести победу!
Откройте для себя революцию в мире ставок с новейшей версией приложения БК Олимп для Android! [url=https://free-apk-olimp.ru/]Скачать Olimp bet[/url] сегодня и сейчас. Мы полностью переработали приложение, обеспечивая неимоверную простоту использования и быстроту загрузки. Теперь разнообразие спортивных событий находится у вас под рукой с улучшенным дизайном и интуитивной навигацией. Прочувствуйте на себе простоту ведения финансов и сделайте каждую ставку незабываемым моментом. Загрузите это обновление сегодня и выходите на новый уровень ставок с БК Олимп, где каждый выбор может принести успех!
ModaOK считается лучшим источником информации о стрижках, татуировках, моде и другое. Здесь вы найдете полезные советы, как вещи сочетать и образы создавать. Также здесь есть лунный календарь. Погрузитесь вместе с нами в fashion-индустрию. https://modaok.ru – сайт, предназначен для тех, кто хочет быть стильной. Здесь вы почерпнете идеи для создания маникюра, сможете узнать, с чем носить черный пиджак, какие мужские и женские часы в моде, как выбрать бюстгалтер, что такое фэмили лук и другое. Вас ждет только самое интересное и свежее!
На сайте https://auto-arenda-anapa.ru/ вам предлагается доступная услуга по прокату авто. Широкий выбор автомобилей даст возможность прямо из окна насладиться комфортами Анапы. Если вы любите путешествовать, лучшим решением будет аренда авто без водителя. Вы можете свободно перемещаться по местности и не зависеть от какого-либо транспорта. Все автомобили проходят техническое обслуживание. Чтобы взять авто напрокат, выберите модель на портале и свяжитесь по телефону с диспетчером. Вам понравится сервис с привлекательными ценами и без ограничений по пробегу.
Откройте для себя новшество в мире ставок с новейшей версией приложения БК Олимп для Android! [url=https://free-apk-olimp.ru/]Приложение Олимп бет на андроид[/url] прямо сейчас. Мы освежили приложение, обеспечивая неимоверную простоту использования и быстроту загрузки. Теперь разнообразие спортивных событий находится у вас под рукой с улучшенным дизайном и интуитивной навигацией. Испытайте на себе простоту ведения финансов и сделайте каждую ставку незабываемым моментом. Установите это приложение сегодня и поднимайте свой опыт ставок с БК Олимп, где каждый выбор может принести победу!
Откройте для себя революцию в мире ставок с новейшей версией приложения БК Олимп для Android! [url=https://free-apk-olimp.ru/]БК Олимп для андроид[/url] прямо сейчас. Мы освежили приложение, обеспечивая неимоверную простоту использования и быстроту загрузки. Теперь выбор соревнований находится у вас под рукой с улучшенным дизайном и интуитивной навигацией. Прочувствуйте на себе простоту ведения финансов и сделайте каждую ставку незабываемым моментом. Загрузите это приложение сегодня и выходите на новый уровень ставок с БК Олимп, где каждый выбор может принести победу!
Нові тренди в світі тактичних кросівок
тактичні чорні кросівки [url=https://vijskovikrosivkifvgh.kiev.ua/]https://vijskovikrosivkifvgh.kiev.ua/[/url] .
На сайте https://container-platform.ru имеется номер телефона для того, чтобы заказать качественные, надежные, практичные контейнерные площадки, предназначенные для мусора. Есть как на 2, так и 3 контейнера. Прямо сейчас ознакомьтесь с расценками на стандартные варианты. Напротив каждого варианта указаны технические характеристики и другая важная информация, которая необходима для того, чтобы быстрее определиться с выбором. Наличие контейнерных площадок обязательно для всех дворов.
[url=http://metformin.store/]generic metformin price[/url]
Познакомьтесь с свежим обновлением приложения БК Олимп для Android, преобразующим ваш опыт в мире ставок! [url=https://olimp-bet-app.ru/]Скачать Олимп бет[/url] доступны всем играющим. Передовой дизайн и ускоренная обработка данных обеспечивают легкий доступ к множеству спортивных событий. Настройте свой аккаунт с непревзойденной легкостью и навигацией, превращая каждую ставку в захватывающий опыт. Загрузите сейчас и начните получать удовольствие от ставок на новом уровне с БК Олимп, где каждое событие – это шанс на успех!
На сайте https://xakexpert.com/ воспользуйтесь услугами высококлассных, компетентных хакеров, которые смогут выполнить все необходимые работы, несмотря на их сложность. Вам доступна такая услуга, как взлом электронной почты, мобильного телефона, а также социальных сетей. Если вы подобрали какого-либо специалиста, то необходимо обязательно с ним связаться. Для этого напишите в чат. Специалист отличается колоссальным опытом, а самое главное, что обращение останется конфиденциальным.
Познакомьтесь с новейшим обновлением приложения БК Олимп для Android, преобразующим ваш опыт в мире ставок! [url=https://olimp-bet-app.ru/]Olimp bet приложение[/url] доступны сегодня для каждого. Передовой дизайн и ускоренная обработка данных обеспечивают легкий доступ к множеству спортивных событий. Кастомизируйте свой аккаунт с непревзойденной легкостью и навигацией, превращая каждую ставку в волнующее приключение. Загрузите прямо сегодня и начните наслаждаться от ставок на новом уровне с БК Олимп, где каждое событие – это шанс на выигрыш!
На сайте https://compsch.com/ собраны самые интересные, увлекательные и содержательные советы на тему компьютеров. Так вы узнаете о том, как правильно оптимизировать, настроить, обслуживать машину. Это позволит технике прослужить намного дольше. Также имеются ценные советы о том, как справиться с вредоносным ПО. Есть информация и об 1С бухгалтерии, о ремонте телевизоров и многое другое, что позволит быстро справиться с ситуацией. Есть данные и о том, как правильно подобрать ноутбук, какие моменты следует учесть при его покупке.
Познакомьтесь с свежим обновлением приложения БК Олимп для Android, преобразующим ваш опыт в мире ставок! [url=https://olimp-bet-app.ru/]Скачать бесплатно Olimp на андроид[/url] доступны всем игрокам. Передовой дизайн и ускоренная обработка данных гарантируют легкий доступ к широкому ассортименту спортивных событий. Кастомизируйте свой аккаунт с непревзойденной легкостью и навигацией, превращая каждую ставку в захватывающий опыт. Скачайте прямо сегодня и начните получать удовольствие от ставок на новом уровне с БК Олимп, где каждое событие – это шанс на выигрыш!
Познакомьтесь с свежим обновлением приложения БК Олимп для Android, изменяющим ваш опыт в мире ставок! [url=https://olimp-bet-app.ru/]Бесплатно скачать Олимп[/url] доступны для всех ставочников. Инновационный дизайн и ускоренная обработка данных гарантируют легкий доступ к множеству спортивных событий. Настройте свой аккаунт с непревзойденной легкостью и навигацией, превращая каждую ставку в захватывающий опыт. Загрузите прямо сегодня и начните получать удовольствие от ставок на новом уровне с БК Олимп, где каждое событие – это шанс на успех!
Реальные кейсы
12. API: углубленное понимание принципа работы
user example com [url=https://www.apiaccess.ru#user-example-com]https://www.apiaccess.ru[/url] .
Встречайте новинку от БК Олимп для Android, которое полностью преобразит ваш подход к ставкам! С переделанным интерфейсом и оптимизированной работой, вы обретете доступ к огромному количеству спортивных мероприятий буквально в несколько касаний. [url=https://olimp-bet-apk.ru/]Скачать Олимп[/url] доступны для каждого. Простое управление аккаунтом и интуитивная навигация сделают процесс ставок удовольствием. Не упустите возможность перейти на новый уровень уже сегодня и откройте секреты успешных ставок с БК Олимп, где каждый ваш выбор может привести к победе!
Встречайте новинку от БК Олимп для Android, которое кардинально изменит ваш подход к ставкам! С усовершенствованным интерфейсом и оптимизированной работой, вы обретете доступ к огромному количеству спортивных мероприятий буквально в несколько касаний. [url=https://olimp-bet-apk.ru/]Приложение Olimp[/url] доступны для игроков. Простое управление аккаунтом и понятная навигация сделают процесс ставок удовольствием. Не упустите возможность перейти на новый уровень уже сегодня и откройте секреты успешных ставок с БК Олимп, где каждый ваш выбор может привести к победе!
На сайте https://dom-otmostka.ru/ оставьте заявку для того, чтобы заказать такую популярную услугу, как монтаж отмостки из брусчатки, бетона, плитки, камня. Кроме того, оказываются услуги, связанные с обустройством ливневой канализации, а также дренажом. В компании работают компетентные сотрудники, которые произведут монтаж дорожек, детских, парковочных площадок. На любые работы действуют гарантии 3 года. Воспользуйтесь и вы компетентными работами лучших сотрудников. На все услуги установлены привлекательные расценки.
Встречайте новинку от БК Олимп для Android, которое кардинально изменит ваш подход к ставкам! С усовершенствованным интерфейсом и ускоренной работой, вы обретете доступ к огромному количеству спортивных мероприятий буквально в несколько касаний. [url=https://olimp-bet-apk.ru/]Скачать сайт Олимп[/url] доступны для каждого. Простое управление аккаунтом и интуитивная навигация сделают процесс ставок удовольствием. Не упустите возможность обновиться уже сегодня и откройте секреты успешных ставок с БК Олимп, где каждый ваш выбор может привести к успеху!
Встречайте новинку от БК Олимп для Android, которое кардинально изменит ваш подход к ставкам! С усовершенствованным интерфейсом и ускоренной работой, вы обретете доступ к огромному количеству спортивных мероприятий буквально в несколько касаний. [url=https://olimp-bet-apk.ru/]Скачать приложение Олимп бет[/url] доступны для игроков. Простое управление аккаунтом и интуитивная навигация сделают процесс ставок удовольствием. Не упустите возможность обновиться уже сегодня и откройте секреты успешных ставок с БК Олимп, где каждый ваш выбор может привести к успеху!
Встречайте новинку от БК Олимп для Android, которое кардинально изменит ваш подход к ставкам! С усовершенствованным интерфейсом и ускоренной работой, вы обретете доступ к бесконечному множеству спортивных мероприятий буквально в несколько касаний. [url=https://olimp-bet-apk.ru/]Приложение букмекера Олимп[/url] доступны для всех. Легкое управление аккаунтом и интуитивная навигация сделают процесс ставок удовольствием. Не упустите возможность перейти на новый уровень уже сегодня и откройте секреты успешных ставок с БК Олимп, где каждый ваш выбор может привести к победе!
Добро пожаловать на сайт https://keywordbox.ru/ где вы найдете самую обширную базу слов в интернете, разобранных на слоги! Мы предоставляем огромный словарь, где каждый может бесплатно воспользоваться возможностью разбирать слова по слогам. Узнайте структуру любого слова и улучшите свои навыки чтения и правописания. Присоединяйтесь к нам сегодня и откройте для себя мир русского языка во всей его красе.
Анонсируем запуск новой версии мобильного приложения БК Лига Ставок для Android! Этот выпуск существенно улучшает ваш досуг с ставками, делая его ещё удобнее и динамичным. [url=https://ligastavok-android.ru/]Скачать приложение бк лига ставок на андроид[/url] уже сегодня! В обновлении вы найдете прямой доступ к огромному ассортименту спортивных мероприятий из любой точки с помощью вашего устройства. Улучшенное управление профилем, инновационная концепция дизайна для удобной навигации и значительно более высокая скорость работы приложения – всё это создано для вас. Станьте частью довольных пользователей и получайте удовольствие от ставок в любой точке планеты и в любое время. Установите обновление приложения БК Лига Ставок уже сегодня и выходите на новый уровень игры!
Анонсируем запуск обновленной мобильного приложения БК Лига Ставок для Android! Этот выпуск полностью преобразует ваш пользовательский опыт, делая его ещё удобнее и динамичным. [url=https://ligastavok-android.ru/]Скачать лига ставок официальный сайт[/url] для всех пользователей! В обновлении вы найдете свободу доступа к многообразию спортивных мероприятий из любой точки с помощью вашего устройства. Улучшенное управление профилем, передовая концепция дизайна для простой навигации и увеличенная скорость работы приложения – всё это создано для вас. Станьте частью довольных пользователей и наслаждайтесь возможностями от ставок где только захотите и в любой момент. Скачайте обновление приложения БК Лига Ставок прямо сейчас и переходите к новому этапу игры!
Анонсируем запуск новой версии мобильного приложения БК Лига Ставок для Android! Этот шаг полностью преобразует ваш пользовательский опыт, делая его более понятным и динамичным. [url=https://ligastavok-android.ru/]Лига ставок официальный скачать бесплатно[/url] для игроков без исключения! В обновлении вы найдете свободу доступа к огромному ассортименту спортивных мероприятий прямо с вашего смартфона. Усовершенствованное управление профилем, передовая концепция дизайна для удобной навигации и значительно более высокая скорость работы приложения – всё это создано для вас. Присоединяйтесь к числу воодушевленных пользователей и наслаждайтесь возможностями от ставок в любой точке планеты и в любой момент. Установите обновление приложения БК Лига Ставок прямо сейчас и выходите на новый уровень игры!
Рады представить выход новой версии мобильного приложения БК Лига Ставок для Android! Этот выпуск существенно улучшает ваш пользовательский опыт, делая его ещё удобнее и динамичным. [url=https://ligastavok-android.ru/]Скачать new лига ставок[/url] для всех сейчас! В обновлении вы найдете прямой доступ к многообразию спортивных мероприятий прямо с вашего смартфона. Улучшенное управление профилем, инновационная концепция дизайна для простой навигации и увеличенная скорость работы приложения – всё это создано для вас. Присоединяйтесь к числу воодушевленных пользователей и получайте удовольствие от ставок в любой точке планеты и в любое время. Скачайте обновление приложения БК Лига Ставок прямо сейчас и выходите на новый уровень ставок!
Анонсируем выход обновленной мобильного приложения БК Лига Ставок для Android! Этот выпуск существенно улучшает ваш пользовательский опыт, делая его ещё удобнее и эффективным. [url=https://ligastavok-android.ru/]Скачать и установить лига ставок на андроид[/url] уже сегодня! В обновлении вы найдете прямой доступ к огромному ассортименту спортивных мероприятий из любой точки с помощью вашего устройства. Улучшенное управление профилем, инновационная концепция дизайна для простой навигации и увеличенная скорость работы приложения – всё это создано для вас. Присоединяйтесь к числу воодушевленных пользователей и наслаждайтесь возможностями от ставок в любой точке планеты и в любой момент. Установите обновление приложения БК Лига Ставок прямо сейчас и выходите на новый уровень игры!
Анонсируем выход новой версии мобильного приложения БК Лига Ставок для Android! Этот выпуск полностью преобразует ваш пользовательский опыт, делая его ещё удобнее и динамичным. [url=https://ligastavok-android.ru/]Скачать лига ставок на телефон[/url] немедленно! В обновлении вы найдете свободу доступа к огромному ассортименту спортивных мероприятий из любой точки с помощью вашего устройства. Улучшенное управление профилем, передовая концепция дизайна для удобной навигации и значительно более высокая скорость работы приложения – всё это создано для вас. Присоединяйтесь к числу воодушевленных пользователей и получайте удовольствие от ставок в любой точке планеты и в любой момент. Установите обновление приложения БК Лига Ставок уже сегодня и переходите к новому этапу ставок!
Рады представить выход обновленной мобильного приложения БК Лига Ставок для Android! Этот шаг полностью преобразует ваш пользовательский опыт, делая его ещё удобнее и динамичным. [url=https://ligastavok-android.ru/]Скачать новое приложение лига ставок[/url] немедленно! В обновлении вы найдете свободу доступа к многообразию спортивных мероприятий прямо с вашего смартфона. Усовершенствованное управление профилем, передовая концепция дизайна для простой навигации и значительно более высокая скорость работы приложения – всё это создано для вас. Присоединяйтесь к числу довольных пользователей и наслаждайтесь возможностями от ставок где только захотите и в любое время. Установите обновление приложения БК Лига Ставок прямо сейчас и переходите к новому этапу игры!
Рады представить выход новой версии мобильного приложения БК Лига Ставок для Android! Этот выпуск существенно улучшает ваш пользовательский опыт, делая его ещё удобнее и динамичным. [url=https://ligastavok-android.ru/]Бк лига ставок скачать бесплатно[/url] для всех пользователей! В обновлении вы найдете свободу доступа к многообразию спортивных мероприятий прямо с вашего смартфона. Усовершенствованное управление профилем, инновационная концепция дизайна для удобной навигации и значительно более высокая скорость работы приложения – всё это создано для вас. Станьте частью довольных пользователей и получайте удовольствие от ставок где только захотите и в любой момент. Установите обновление приложения БК Лига Ставок прямо сейчас и переходите к новому этапу игры!
Анонсируем выход новой версии мобильного приложения БК Лига Ставок для Android! Этот шаг полностью преобразует ваш досуг с ставками, делая его более понятным и динамичным. [url=https://ligastavok-android.ru/]Лига ставок скачать на телефон бесплатно андроид[/url] сейчас же! В обновлении вы найдете прямой доступ к огромному ассортименту спортивных мероприятий прямо с вашего смартфона. Улучшенное управление профилем, инновационная концепция дизайна для простой навигации и значительно более высокая скорость работы приложения – всё это создано для вас. Станьте частью довольных пользователей и наслаждайтесь возможностями от ставок в любой точке планеты и в любой момент. Скачайте обновление приложения БК Лига Ставок уже сегодня и переходите к новому этапу игры!
[url=http://olisinopril.com/]order cheap lisinopril[/url]
Каталог салонов эпиляции https://findepil.ru/ Москвы и Московской области. Химки, Люберцы, Красногорск, Зеленоград и т.д. Все виды эпиляции – электроэпиляция, депиляция воском, шугаринг, лазерная эпиляция. Можно выбрать салон по цене, для примера выбраны стандартные процедуры эпиляции – руки воск, ноги шугаринг. Каталог постоянно пополняется.
Вашему вниманию предлагается эксклюзивное предложение от БК Лига Ставок – фрибет для новых игроков! Это фрибет даёт возможность вам испытать удачу без риска потери собственных средств, предоставляя широкие возможности для получения призов с первых же шагов в мире ставок. [url=https://ligastavok-freebet.ru/]Лига ставок получить фрибет[/url] для всех новичков после регистрации! С фрибетом от БК Лига Ставок, вы имеете возможность испытать все преимущества ставок без каких-либо финансовых обязательств. Это идеальный старт для тех, кто хочет стать частью мира спортивных ставок с минимальными рисками. Не упускайте свой шанс и начните своё приключение в БК Лига Ставок с нашим фрибетом. Заберите свой фрибет сейчас же и откройте для себя мир ставок без рисков!
Вашему вниманию предлагается эксклюзивное предложение от БК Лига Ставок – фрибет для новых игроков! Это бесплатная ставка позволяет вам испытать удачу без риска потери собственных средств, открывая новые горизонты для выигрыша с первых же шагов в мире ставок. [url=https://ligastavok-freebet.ru/]Получить фрибет лига ставок[/url] для всех новичков после регистрации! С фрибетом от БК Лига Ставок, вы имеете возможность испытать все преимущества ставок без каких-либо финансовых обязательств. Это идеальный старт для тех, кто хочет стать частью мира спортивных ставок с минимальными рисками. Не упускайте свой шанс и сделайте первый шаг к большим выигрышам в БК Лига Ставок с нашим фрибетом. Заберите свой фрибет уже сегодня и откройте для себя мир ставок без рисков!
Знакомьтесь с эксклюзивное предложение от БК Лига Ставок – фрибет для новых игроков! Это фрибет даёт возможность вам испытать удачу без риска потери собственных средств, предоставляя широкие возможности для получения призов с первых же шагов в мире ставок. [url=https://ligastavok-freebet.ru/]Фрибет лига ставок[/url] для всех новичков после регистрации! С фрибетом от БК Лига Ставок, вы имеете возможность ознакомиться с платформой без каких-либо финансовых обязательств. Это отличный способ начать для тех, кто хочет стать частью мира спортивных ставок с минимальными рисками. Не упускайте свой шанс и начните своё приключение в БК Лига Ставок с нашим фрибетом. Активируйте ваш фрибет сейчас же и стартуйте к выигрышам!
Знакомьтесь с эксклюзивное предложение от БК Лига Ставок – фрибет для новых игроков! Это бесплатная ставка даёт возможность вам сделать ставку без риска потери собственных средств, открывая новые горизонты для получения призов с первых же шагов в мире ставок. [url=https://ligastavok-freebet.ru/]Лига ставок фрибет за пополнение[/url] для всех новичков после регистрации! С фрибетом от БК Лига Ставок, вы имеете возможность испытать все преимущества ставок без каких-либо финансовых обязательств. Это идеальный старт для тех, кто хочет погрузиться в мир ставок с минимальными рисками. Воспользуйтесь этим предложением и сделайте первый шаг к большим выигрышам в БК Лига Ставок с нашим фрибетом. Активируйте ваш фрибет сейчас же и стартуйте к выигрышам!
На сайте https://www.kapatel.ru/ вы почерпнете много интересной, познавательной информации, которая касается дачи, сада, а также огорода. Вы узнаете о том, как правильно обустроить дачу, огород так, чтобы они были функциональными, вместительными и радовали богатым урожаем. Имеются данные о строительстве, ремонте и различных отделочных работах. Опубликованы материалы о вредителях, различных заболеваниях, о самых цветущих растениях. Вы можете задавать вопросы и присылать сообщения для уточнения некоторых моментов.
Знакомьтесь с эксклюзивное предложение от БК Лига Ставок – фрибет для новых игроков! Это фрибет позволяет вам испытать удачу без риска потери собственных средств, предоставляя широкие возможности для выигрыша с первых же шагов в мире ставок. [url=https://ligastavok-freebet.ru/]Лига ставок фрибет за регистрацию без депозита получить[/url] новым пользователям после регистрации! С фрибетом от БК Лига Ставок, вы имеете возможность ознакомиться с платформой без каких-либо финансовых обязательств. Это идеальный старт для тех, кто хочет погрузиться в мир ставок с минимальными рисками. Воспользуйтесь этим предложением и начните своё приключение в БК Лига Ставок с нашим фрибетом. Заберите свой фрибет сейчас же и откройте для себя мир ставок без рисков!
На сайте https://shemi-otopleniya.ru/ ознакомьтесь с техническими характеристиками, особенностями, нюансами гибкой подводки, выполненной из нержавеющей стали. Так вы сможете ознакомиться с размерами, диаметром, а также различными модификациями. Есть варианты небольших размеров, а также более внушительных. Также можно ознакомиться и с информацией, которая посвящена шаровому крану. Регулярно публикуются любопытные и интересные новости на данную тематику. Обязательно ознакомьтесь и с фотографиями.
Вашему вниманию предлагается эксклюзивное предложение от БК Лига Ставок – фрибет для новых игроков! Это фрибет даёт возможность вам сделать ставку без риска потери собственных средств, открывая широкие возможности для выигрыша с первых же шагов в мире ставок. [url=https://ligastavok-freebet.ru/]Лига ставок фрибет 2222[/url] для всех новичков после регистрации! С фрибетом от БК Лига Ставок, вы имеете возможность испытать все преимущества ставок без каких-либо финансовых обязательств. Это идеальный старт для тех, кто хочет стать частью мира спортивных ставок с минимальными рисками. Воспользуйтесь этим предложением и сделайте первый шаг к большим выигрышам в БК Лига Ставок с нашим фрибетом. Активируйте ваш фрибет уже сегодня и стартуйте к выигрышам!
Знакомьтесь с эксклюзивное предложение от БК Лига Ставок – фрибет для новых игроков! Это фрибет даёт возможность вам сделать ставку без риска потери собственных средств, предоставляя широкие возможности для получения призов с первых же шагов в мире ставок. [url=https://ligastavok-freebet.ru/]Фрибет лига ставок на сегодня бесплатно[/url] новым пользователям после регистрации! С фрибетом от БК Лига Ставок, вы имеете возможность испытать все преимущества ставок без каких-либо финансовых обязательств. Это идеальный старт для тех, кто хочет стать частью мира спортивных ставок с минимальными рисками. Не упускайте свой шанс и сделайте первый шаг к большим выигрышам в БК Лига Ставок с нашим фрибетом. Заберите свой фрибет уже сегодня и откройте для себя мир ставок без рисков!
Знакомьтесь с эксклюзивное предложение от БК Лига Ставок – фрибет для новых игроков! Это фрибет даёт возможность вам сделать ставку без риска потери собственных средств, открывая широкие возможности для получения призов с первых же шагов в мире ставок. [url=https://ligastavok-freebet.ru/]Лигаставок фрибет[/url] для всех новичков после регистрации! С фрибетом от БК Лига Ставок, вы имеете возможность испытать все преимущества ставок без каких-либо финансовых обязательств. Это отличный способ начать для тех, кто хочет стать частью мира спортивных ставок с минимальными рисками. Не упускайте свой шанс и начните своё приключение в БК Лига Ставок с нашим фрибетом. Заберите свой фрибет уже сегодня и стартуйте к выигрышам!
Вашему вниманию предлагается эксклюзивное предложение от БК Лига Ставок – фрибет для новых игроков! Это фрибет позволяет вам сделать ставку без риска потери собственных средств, предоставляя новые горизонты для выигрыша с первых же шагов в мире ставок. [url=https://ligastavok-freebet.ru/]Лига ставок фрибет за регистрацию условия[/url] новым пользователям после регистрации! С фрибетом от БК Лига Ставок, вы имеете возможность испытать все преимущества ставок без каких-либо финансовых обязательств. Это отличный способ начать для тех, кто хочет стать частью мира спортивных ставок с минимальными рисками. Воспользуйтесь этим предложением и начните своё приключение в БК Лига Ставок с нашим фрибетом. Активируйте ваш фрибет уже сегодня и стартуйте к выигрышам!
The calculator on a smartphone is very constrained in functions. As an architect, I often require a scientific online calculator, such as this https://calculator-online.info/. It is effective for various tasks like financial calculations, percentage calculations, solving complex equations, finding square and cube roots, and more
Рады сообщить о доступности эксклюзивного промокода для БК Лига Ставок! Этот уникальный код предоставляет доступ к эксклюзивным привилегиям и усиливает ваш опыт ставок, делая его более прибыльным и волнующим. [url=https://ligastavok-promocode.ru/]Промокод на регистрацию лига ставок[/url] для всех новых пользователей! С данным промокодом, игроки получают непосредственный доступ к бонусным средствам, привилегиям при регистрации и эксклюзивным акциям. Не упустите шанс стать частью элитного клуба пользователей БК Лига Ставок и максимизируйте свои выигрыши с нашим промокодом. Используйте код уже сегодня и заберите свои бонусы еще до первой ставки!
Представляем выпуске эксклюзивного промокода для БК Лига Ставок! Этот уникальный код предоставляет доступ к эксклюзивным привилегиям и повышает ваш игровой процесс, делая его более прибыльным и волнующим. [url=https://ligastavok-promocode.ru/]Промокод для лига ставок[/url] для всех новых пользователей! С данным промокодом, игроки получают непосредственный доступ к бонусным средствам, ускоренной процедуре регистрации и специальным предложениям. Воспользуйтесь этой возможностью стать частью элитного клуба пользователей БК Лига Ставок и увеличьте свои шансы на успех с нашим промокодом. Активируйте промокод уже сегодня и начните выигрывать еще до первой ставки!
Представляем доступности эксклюзивного промокода для БК Лига Ставок! Этот специальный промокод предоставляет доступ к особым бонусам и усиливает ваш опыт ставок, делая его более прибыльным и волнующим. [url=https://ligastavok-promocode.ru/]Промокод на бесплатную ставку лига ставок[/url] для всех новых пользователей! С его использованием, игроки получают доступ к бонусным средствам, ускоренной процедуре регистрации и эксклюзивным акциям. Воспользуйтесь этой возможностью стать частью элитного клуба пользователей БК Лига Ставок и увеличьте свои шансы на успех с нашим промокодом. Активируйте промокод прямо сейчас и начните выигрывать еще до первой ставки!
Рады сообщить о выпуске эксклюзивного промокода для БК Лига Ставок! Этот уникальный код открывает доступ к эксклюзивным привилегиям и повышает ваш игровой процесс, делая его ещё более выгодным и захватывающим. [url=https://ligastavok-promocode.ru/]Лига ставок купон[/url] для активации сегодня! С его использованием, игроки получают непосредственный доступ к дополнительным бонусам на ставки, ускоренной процедуре регистрации и эксклюзивным акциям. Не упустите шанс стать частью привилегированной группы пользователей БК Лига Ставок и максимизируйте свои выигрыши с нашим промокодом. Используйте код прямо сейчас и начните выигрывать еще до первой ставки!
Представляем доступности эксклюзивного промокода для БК Лига Ставок! Этот специальный промокод предоставляет доступ к особым бонусам и усиливает ваш опыт ставок, делая его ещё более выгодным и волнующим. [url=https://ligastavok-promocode.ru/]Рабочий промокод лига ставок[/url] сразу после активации! С данным промокодом, игроки получают непосредственный доступ к бонусным средствам, ускоренной процедуре регистрации и эксклюзивным акциям. Не упустите шанс стать частью элитного клуба пользователей БК Лига Ставок и максимизируйте свои выигрыши с нашим промокодом. Используйте код прямо сейчас и начните выигрывать еще до первой ставки!
Представляем доступности эксклюзивного промокода для БК Лига Ставок! Этот специальный промокод открывает доступ к особым бонусам и усиливает ваш опыт ставок, делая его ещё более выгодным и волнующим. [url=https://ligastavok-promocode.ru/]Промокод лига ставок на сегодня на фрибет 500[/url] после ввода кода! С его использованием, игроки получают доступ к дополнительным бонусам на ставки, ускоренной процедуре регистрации и специальным предложениям. Не упустите шанс стать частью привилегированной группы пользователей БК Лига Ставок и максимизируйте свои выигрыши с нашим промокодом. Используйте код прямо сейчас и начните выигрывать еще до первой ставки!
Самый ожидаемый футбольный поединок сезона – Манчестер Сити против Реала Мадрид! Энтузиазм, волнение, и адреналин ждут нас на поле. Но этот день может быть еще более увлекательным благодаря промокоду PLAYDAY от 1win! Ставьте на своего героя с уверенностью и удвойте свои шансы на победу! Не упустите возможность – забирайте [url=https://www.pinterest.com/pin/916693699143021366/]Манчестер Сити Реал Мадрид промокод[/url] уже сегодня!
Самый ожидаемый футбольный поединок сезона – Манчестер Сити против Реала Мадрид! Энтузиазм, напряжение, и невероятные эмоции ждут нас на поле. Но этот день может быть еще более интригующим благодаря промокоду PLAYDAY от 1win! Ставьте на своего героя с уверенностью и повысьте свои шансы на победу! Не упустите возможность – забирайте [url=https://www.pinterest.com/pin/916693699143021366/]Манчестер Сити Реал промокод на депозит[/url] уже сегодня!
Самый ожидаемый футбольный поединок сезона – Манчестер Сити против Реала Мадрид! Азарт, волнение, и адреналин ждут нас на поле. Но этот день может быть еще более увлекательным благодаря промокоду PLAYDAY от 1win! Ставьте на своего героя с уверенностью и удвойте свои шансы на победу! Не упустите шанс – забирайте [url=https://www.pinterest.com/pin/916693699143021366/]Манчестер Сити Реал бонус[/url] уже сегодня!
Самый ожидаемый футбольный поединок сезона – Манчестер Сити против Реала Мадрид! Азарт, нервы, и невероятные эмоции ждут нас на поле. Но этот день может быть еще более интригующим благодаря промокоду PLAYDAY от 1win! Ставьте на своего любимца с уверенностью и повысьте свои шансы на победу! Не упустите шанс – забирайте [url=https://www.pinterest.com/pin/916693699143021366/]Манчестер Сити Реал 500 за депозит[/url] уже сегодня!
Самый ожидаемый футбольный поединок сезона – Манчестер Сити против Реала Мадрид! Энтузиазм, нервы, и невероятные эмоции ждут нас на поле. Но этот день может быть еще более захватывающим благодаря промокоду PLAYDAY от 1win! Ставьте на своего любимца с уверенностью и повысьте свои шансы на победу! Не упустите возможность – забирайте [url=https://www.pinterest.com/pin/916693699143021366/]Манчестер Сити Реал Мадрид промокод на депозит[/url] уже сегодня!
[url=https://prednisoneo.com/]how much is prednisone cost[/url]
Быстрая доставка
– Кран для радиатора отопления: сравнение и покупка
кран шаровой это [url=https://krany-sharovye-nerzhaveyushie-msk.ru/]кран шаровой это[/url] .
На сайте https://vk.com/promocodi.yandex.market вы найдете промокоды Яндекс.Маркета. Они предоставят вам отличную возможность сэкономить приличную сумму денег. Новые промокоды публикуются в группе ежедневно, чтобы ими воспользовались все желающие. Также есть выгодные предложения и от таких популярных и надежных магазинов, как «Самокат», «Спортмастер». Используйте промокод каждый раз при оформлении. Прямо сейчас есть возможность использовать промокод, чтобы получить выгоду в 200 рублей.
Самый ожидаемый футбольный поединок сезона – Манчестер Сити против Реала Мадрид! Энтузиазм, волнение, и невероятные эмоции ждут нас на поле. Но этот день может быть еще более интригующим благодаря промокоду PLAYDAY от 1win! Ставьте на своего героя с уверенностью и растите свои шансы на победу! Не упустите возможность – забирайте [url=https://www.pinterest.com/pin/916693699143021366/]Манчестер Сити Реал Мадрид бонус 500[/url] уже сегодня!
Самый ожидаемый футбольный поединок сезона – Манчестер Сити против Реала Мадрид! Азарт, нервы, и невероятные эмоции ждут нас на поле. Но этот день может быть еще более захватывающим благодаря промокоду PLAYDAY от 1win! Ставьте на своего любимца с уверенностью и растите свои шансы на победу! Не упустите возможность – забирайте [url=https://www.pinterest.com/pin/916693699143021366/]Манчестер Сити Реал 500 за депозит[/url] уже сегодня!
https://praktikaotchet.ru/
Самый ожидаемый футбольный поединок сезона – Манчестер Сити против Реала Мадрид! Энтузиазм, нервы, и ожидание ждут нас на поле. Но этот день может быть еще более захватывающим благодаря промокоду PLAYDAY от 1win! Ставьте на своего любимца с уверенностью и растите свои шансы на победу! Не упустите шанс – забирайте [url=https://www.pinterest.com/pin/916693699143021366/]Манчестер Сити Реал бонус 500[/url] уже сегодня!
Битва гигантов на поле – Бавария против Арсенала! Эмоции, напряжение и уникальные ситуации ждут нас в этом поединке. Но почему бы не добавить еще больше интриги с помощью промокода PLAYDAY от 1win? Сделайте ставку на победителя и повысьте свои выигрышные возможности! Не упустите этот шанс – воспользуйтесь [url=https://www.pinterest.com/pin/916693699143021391]Бавария Арсенал бонус код за регистрацию[/url] уже сегодня и получайте удовольствие от игры в полную силу!
Битва гигантов на поле – Бавария против Арсенала! Волнение, азарт и невероятные моменты ждут нас в этом противостоянии. Но почему бы не добавить еще больше интриги с помощью промокода PLAYDAY от 1win? Сделайте ставку на победителя и растите свои вероятность успеха! Не упустите этот шанс – воспользуйтесь [url=https://www.pinterest.com/pin/916693699143021391]Бавария Арсенал бонус код за регистрацию[/url] уже сегодня и получайте удовольствие от игры в полную силу!
Битва гигантов на поле – Бавария против Арсенала! Адреналин, азарт и уникальные ситуации ждут нас в этом противостоянии. Но почему бы не добавить еще больше эмоций с помощью промокода PLAYDAY от 1win? Сделайте ставку на победный исход и растите свои выигрышные возможности! Не упустите этот возможность – воспользуйтесь [url=https://www.pinterest.com/pin/916693699143021391]Бавария Арсенал промокод[/url] уже сегодня и радуйтесь игры в полную силу!
Битва гигантов на поле – Бавария против Арсенала! Волнение, азарт и невообразимые моменты ждут нас в этом противостоянии. Но почему бы не добавить еще больше интриги с помощью промокода PLAYDAY от 1win? Сделайте ставку на победную команду и увеличьте свои шансы на победу! Не упустите этот шанс – используйте [url=https://www.pinterest.com/pin/916693699143021391]Бавария Арсенал промокод на депозит[/url] уже сегодня и наслаждайтесь игры в полную силу!
Битва гигантов на поле – Бавария против Арсенала! Эмоции, азарт и уникальные ситуации ждут нас в этом противостоянии. Но почему бы не добавить еще больше интриги с помощью промокода PLAYDAY от 1win? Сделайте ставку на победную команду и повысьте свои выигрышные возможности! Не упустите этот шанс – активируйте [url=https://www.pinterest.com/pin/916693699143021391]Бавария Арсенал бонус[/url] уже сегодня и получайте удовольствие от игры в полную силу!
https://praktikaucheba.ru/
Встречайте спортивное событие на высшем уровне с промокодом PLAYDAY от 1win! Независимо от того, на какой матч вы делаете ставку – будь то спортивное противостояние – этот промокод приносит вам дополнительные шансы для победы. Повысьте свои шансы на успех, применяя промокод PLAYDAY при размещении ставок. Не упустите шанс на потрясающие впечатления и дополнительные призы – воспользуйтесь [url=https://www.pinterest.com/pin/916693699143021313/]1win casino бонусы[/url] прямо сейчас на 1win!
[url=http://azithromycinhq.com/]zithromax purchase online[/url]
На сайте https://1-line.su/ воспользуйтесь помощью квалифицированных, компетентных сотрудников популярного предприятия «Первая линия». Они окажут необходимую поддержку в вопросах оптимизации, автоматизации различных бизнес-процессов. Компания сделает все возможное для того, чтобы вы работали наиболее эффективно и продуктивно. Воспользуйтесь полным набором работающих инструментов, которые точно вам пригодятся для построения бизнеса. Для того чтобы начать сотрудничество, просто напишите на электронную почту.
Встречайте матч на новом уровне с промокодом PLAYDAY от 1win! Независимо от того, на какую матч вы делаете ставку – будь то футбольная битва – этот промокод приносит вам дополнительные шансы для победы. Повысьте свои выигрышные шансы, используя промокод PLAYDAY при размещении ставок. Не упустите возможность на захватывающий опыт и бонусные выигрыши – используйте [url=https://www.pinterest.com/pin/916693699143021313/]промокод 1win при регистрации на сегодня[/url] прямо сейчас на 1win!
Встречайте матч на пике с промокодом PLAYDAY от 1win! Независимо от того, на какую игру вы делаете ставку – будь то футбольная битва – этот промокод приносит вам экстра-шансы для победы. Увеличьте свои вероятность победы, активируя промокод PLAYDAY при размещении ставок. Не упустите шанс на увлекательный опыт и дополнительные выигрыши – активируйте [url=https://www.pinterest.com/pin/916693699143021313/]1вин бонус при регистрации[/url] прямо сейчас на 1win!
Встречайте матч на высшем уровне с промокодом PLAYDAY от 1win! Независимо от того, на какую событие вы ставите – будь то спортивное противостояние – этот промокод приносит вам экстра-шансы для победы. Повысьте свои вероятность победы, активируя промокод PLAYDAY при размещении ставок. Не упустите шанс на потрясающие впечатления и бонусные выигрыши – активируйте [url=https://www.pinterest.com/pin/916693699143021313/]1win casino бонус код[/url] прямо сейчас на 1win!
Встречайте игру на новом уровне с промокодом PLAYDAY от 1win! Независимо от того, на какую матч вы ставите – будь то спортивное противостояние – этот промокод приносит вам экстра-шансы для победы. Повысьте свои выигрышные шансы, активируя промокод PLAYDAY при размещении ставок. Не упустите шанс на потрясающие впечатления и бонусные выигрыши – используйте [url=https://www.pinterest.com/pin/916693699143021313/]промокод при регистрации 1win[/url] прямо сейчас на 1win!
Встречайте игру на высшем уровне с промокодом PLAYDAY от 1win! Независимо от того, на какой матч вы делаете ставку – будь то спортивное противостояние – этот промокод приносит вам экстра-шансы для победы. Расти свои вероятность победы, активируя промокод PLAYDAY при размещении ставок. Не упустите возможность на потрясающие впечатления и бонусные выигрыши – используйте [url=https://www.pinterest.com/pin/916693699143021313/]1win casino бонус код[/url] прямо сейчас на 1win!
[url=https://medicinesaf.online/]online pharmacy birth control pills[/url]
Встречайте игру на новом уровне с промокодом PLAYDAY от 1win! Независимо от того, на какую событие вы ставите – будь то футбольная битва – этот промокод приносит вам дополнительные шансы для победы. Повысьте свои вероятность победы, используя промокод PLAYDAY при размещении ставок. Не упустите шанс на потрясающие впечатления и дополнительные призы – воспользуйтесь [url=https://www.pinterest.com/pin/916693699143021313/]1win промокод на деньги[/url] прямо сейчас на 1win!
https://referatnash.ru/
https://vipbuket64.ru/
Добро пожаловать на сайт https://nauka-slovo.ru/ где вы найдете самую обширную базу слов в интернете, разделенных по корням! Мы предоставляем огромный словарь, где каждый может бесплатно воспользоваться возможностью изучения корней слов. Узнайте состав и происхождение любого слова, расширьте свой лексический запас и глубже погрузитесь в структуру русского языка. Присоединяйтесь к нам сегодня и начните путешествие в мир корней слов!
В настоящее время наши дни могут содержать неожиданные расходы и экономические трудности, и в такие моменты каждый ищет надежную поддержку. На сайте [url=https://brokers-group.ru/]взять деньги в кредит[/url] вам помогут без избыточной бюрократии. В своей роли агента я стремлюсь распространить эту информацию, чтобы помочь людям в сложной ситуации. Наша система обеспечивает прозрачные условия и быструю обработку заявок, чтобы каждый мог решить свои денежные проблемы быстро и без лишних хлопот.
В настоящее время наши дни могут содержать неожиданные расходы и финансовые вызовы, и в такие моменты каждый ищет помощь. На сайте [url=https://brokers-group.ru/]взять денег в долг срочно[/url] вам помогут без избыточной бюрократии. В своей роли представителя я стремлюсь распространить эту информацию, чтобы помочь тем, кто столкнулся с временными трудностями. Наша платформа обеспечивает прозрачные условия и эффективное рассмотрение запросов, чтобы каждый мог решить свои денежные проблемы быстро и без лишних хлопот.
В настоящее время наши дни могут содержать неожиданные расходы и финансовые вызовы, и в такие моменты каждый ищет помощь. На сайте [url=https://brokers-group.ru/]займ на карту без отказа[/url] вам помогут без избыточной бюрократии. В своей роли агента я стремлюсь поделиться этими сведениями, чтобы помочь людям в сложной ситуации. Наша платформа обеспечивает честные условия и быструю обработку заявок, чтобы каждый мог разрешить свои финансовые вопросы быстро и без лишних хлопот.
В настоящее время наши дни могут содержать неожиданные расходы и экономические трудности, и в такие моменты каждый ищет помощь. На сайте [url=https://brokers-group.ru/]деньги в долг[/url] вам помогут без лишних сложностей. В своей роли представителя я стремлюсь поделиться этими сведениями, чтобы помочь людям в сложной ситуации. Наша система обеспечивает честные условия и эффективное рассмотрение запросов, чтобы каждый мог разрешить свои финансовые вопросы быстро и без лишних хлопот.
В настоящее время наши дни могут содержать неожиданные расходы и экономические трудности, и в такие моменты каждый ищет надежную поддержку. На сайте [url=https://brokers-group.ru/]займ на карту без отказа[/url] вам помогут без лишних сложностей. В своей роли агента я стремлюсь поделиться этими сведениями, чтобы помочь тем, кто столкнулся с временными трудностями. Наша платформа обеспечивает прозрачные условия и быструю обработку заявок, чтобы каждый мог решить свои денежные проблемы быстро и без лишних хлопот.
На сайте https://td1000.ru/ находится огромное количество интересных, познавательных статей, которые касаются того, как развить социальные сети и простимулировать количество подписчиков. Также имеются данные о турах по Ямалу. Опубликован материал и о вместительных гардеробных и о том, как подобрать мебель. Есть контент, посвященный различным игровым онлайн-заведениям. Полезные статьи откроют вам глаза на многочисленные вопросы. По этой причине вы узнаете много нового и интересного.
В настоящее время наши дни могут содержать внезапные издержки и финансовые вызовы, и в такие моменты каждый ищет помощь. На сайте [url=https://brokers-group.ru/]деньги в долг в Минске срочно[/url] вам помогут без лишних сложностей. В своей роли агента я стремлюсь поделиться этими сведениями, чтобы помочь людям в сложной ситуации. Наша платформа обеспечивает честные условия и быструю обработку заявок, чтобы каждый мог решить свои денежные проблемы оперативно и без лишних затрат времени.
В настоящее время наши дни могут содержать неожиданные расходы и финансовые вызовы, и в такие моменты каждый ищет помощь. На сайте [url=https://brokers-group.ru/]деньги в кредит[/url] вам помогут без лишних сложностей. В своей роли агента я стремлюсь поделиться этими сведениями, чтобы помочь людям в сложной ситуации. Наша платформа обеспечивает прозрачные условия и быструю обработку заявок, чтобы каждый мог разрешить свои финансовые вопросы оперативно и без лишних затрат времени.
В настоящее время наши дни могут содержать неожиданные расходы и экономические трудности, и в такие моменты каждый ищет помощь. На сайте [url=https://brokers-group.ru/]brokers-group.ru[/url] вам помогут без избыточной бюрократии. В своей роли представителя я стремлюсь поделиться этими сведениями, чтобы помочь людям в сложной ситуации. Наша платформа обеспечивает прозрачные условия и быструю обработку заявок, чтобы каждый мог разрешить свои финансовые вопросы оперативно и без лишних затрат времени.
В настоящее время наши дни могут содержать неожиданные расходы и экономические трудности, и в такие моменты каждый ищет надежную поддержку. На сайте [url=https://brokers-group.ru/]получить займ в Минске[/url] вам помогут без лишних сложностей. В своей роли представителя я стремлюсь поделиться этими сведениями, чтобы помочь тем, кто столкнулся с временными трудностями. Наша система обеспечивает честные условия и эффективное рассмотрение запросов, чтобы каждый мог решить свои денежные проблемы оперативно и без лишних затрат времени.
zeekr 007
li l8
На сайте https://podvodka.okis.ru/ ознакомьтесь с гибкой подводкой различного дюйма. Посмотрите технические характеристики, особенности и другую полезную информацию, которая будет вам необходима. На портале имеется таблица на некоторые размеры. Она поможет лучше сориентироваться в выборе. Также указаны и расценки, которые потребуются для составления сметы. Кроме того, имеется и важная информация относительно проведения профессиональных монтажных работ. Вы узнаете обо всех нюансах, которые потребуются в процессе установки.
В наше время многие из нас сталкиваются с внезапными затратами и денежными трудностями, и в такие моменты важно иметь возможность взять кредит. На сайте [url=https://dengizaimy.ru/]деньги в долг срочно[/url] вам помогут с минимальными усилиями. В качестве официального представителя Брокерс Групп, я рад делиться этой информацией, чтобы помочь людям, кто столкнулся с финансовыми трудностями. Наша платформа гарантирует честные условия и быструю обработку заявок, чтобы каждый мог решить свои финансовые вопросы с минимальными временными затратами.
В наше время многие из нас сталкиваются с внезапными затратами и денежными трудностями, и в такие моменты важно иметь возможность взять кредит. На сайте [url=https://dengizaimy.ru/]dengizaimy.ru[/url] вам помогут быстро и удобно. В качестве представителя Брокерс Групп, я рад делиться этой информацией, чтобы помочь людям, кто нуждается в финансовой поддержке. Наша платформа гарантирует честные условия и оперативное рассмотрение запросов, чтобы каждый мог разрешить свои денежные проблемы с минимальными временными затратами.
В наше время многие из нас сталкиваются с внезапными затратами и экономическими вызовами, и в такие моменты важно иметь возможность взять кредит. На сайте [url=https://dengizaimy.ru/]взять деньги в долг в Минске[/url] вам помогут быстро и удобно. В качестве официального представителя Брокерс Групп, я рад делиться этой важной информацией, чтобы помочь людям, кто столкнулся с финансовыми трудностями. Наша платформа гарантирует честные условия и быструю обработку заявок, чтобы каждый мог решить свои финансовые вопросы с минимальными временными затратами.
На сайте https://climat.best приобретите качественные, функциональные кондиционеры, сплит-системы, тепловые насосы, а также чиллеры. Вся продукция именитых, проверенных и надежных брендов, а потому техника прослужит долго без потери технических характеристик, возможностей. А если необходимо подобрать что-то определенное, то воспользуйтесь специальным поиском. Перед вами есть и самые популярные позиции, которые пользуются особенным спросом среди покупателей. В интернет-магазине вы отыщите как настенные, так и напольно-потолочные конструкции, кассетные.
Циклёвка паркета: особенности и этапы услуги
Циклёвка паркета — это процесс восстановления внешнего вида паркетного пола путём удаления верхнего повреждённого слоя и возвращения ему первоначального вида. Услуга включает в себя несколько этапов:
Подготовка: перед началом работы необходимо защитить мебель и другие предметы от пыли и грязи, а также удалить плинтусы.
Шлифовка: с помощью шлифовальной машины удаляется старый лак и верхний повреждённый слой древесины.
Шпатлёвка: после шлифовки поверхность паркета шпатлюется для заполнения трещин и выравнивания поверхности.
Грунтовка: перед нанесением лака паркет грунтуется для улучшения адгезии и защиты от плесени и грибка.
Нанесение лака: лак наносится в несколько слоёв с промежуточной шлифовкой между ними.
Полировка: после нанесения последнего слоя лака паркет полируется для придания поверхности блеска и гладкости.
Циклёвка паркета позволяет обновить внешний вид пола, восстановить его структуру и продлить срок службы.
Циклёвка паркета
Found an enthralling read that I’d recommend – it’s truly fascinating http://cozy.moibb.ru/viewtopic.php?f=31&t=5275
Качественное написание рефератов https://referatnovy.ru/, курсовых и дипломных работ от лучших авторов. Уникальные работы под ключ. Заказать студенческую работу за 2 дня.
В настоящей эпохе финансовые сложности не редкость, и каждый из нас может оказаться в ситуации, когда необходима дополнительная финансовая поддержка. На сайте [url=https://moneybel.ru/]деньги в долг в Минске[/url] помогут преодолеть эти трудности, предоставляя простой и удобный способ получения кредита. Мы понимаем, что времени на решение проблемы не хватает, поэтому мы обрабатываем заявки оперативно и эффективно. В роли представителя Манибел, мы нацелены на то, чтобы помочь каждому клиенту преодолеть свои денежные трудности, предоставив прозрачные и выгодные условия кредитования. Доверьтесь нашей компании, и мы решим ваши финансовые проблемы.
В настоящей эпохе финансовые трудности не редкость, и каждый из нас может оказаться в ситуации, когда необходима дополнительная финансовая поддержка. На сайте [url=https://moneybel.ru/]деньги в долг срочно[/url] помогут преодолеть эти трудности, принося простой и лёгкий метод займа. Мы понимаем, что время играет ключевую роль, поэтому мы обрабатываем заявки оперативно и эффективно. В роли представителя Манибел, мы нацелены на то, чтобы помочь каждому клиенту преодолеть свои денежные трудности, предложив ясные и выгодные условия займа. Доверьтесь нашей компании, и мы решим ваши финансовые проблемы.
В настоящей эпохе финансовые трудности не редкость, и каждый человек может оказаться в ситуации, когда необходима дополнительная финансовая поддержка. На сайте [url=https://moneybel.ru/]moneybel[/url] помогут решить эти проблемы, предоставляя простой и лёгкий метод получения кредита. Мы осознаём, что время играет ключевую роль, поэтому мы обрабатываем заявки оперативно и эффективно. В роли представителя Манибел, наша цель – помочь каждому клиенту преодолеть финансовые затруднения, предоставив прозрачные и выгодные условия кредитования. Доверьтесь нам, и мы поможем вам в решении ваших финансовых проблем.
В сегодняшнем обществе финансовые трудности не редкость, и каждый из нас может оказаться в ситуации, когда необходима дополнительная финансовая поддержка. На сайте [url=https://moneybel.ru/]быстрый займ[/url] помогут преодолеть эти трудности, предоставляя простой и удобный способ получения кредита. Мы осознаём, что время играет ключевую роль, поэтому мы обрабатываем заявки оперативно и эффективно. В качестве представителя Манибел, мы нацелены на то, чтобы помочь каждому клиенту преодолеть свои денежные трудности, предоставив прозрачные и выгодные условия кредитования. Доверьтесь нашей компании, и мы поможем вам в решении ваших финансовых проблем.
В настоящей эпохе финансовые сложности не редкость, и каждый из нас может оказаться в ситуации, когда необходима дополнительная финансовая поддержка. На сайте [url=https://moneybel.ru/]деньги в долг Минск[/url] помогут решить эти проблемы, принося простой и удобный способ получения кредита. Мы осознаём, что время играет ключевую роль, поэтому наш процесс обработки заявок максимально быстр и эффективен. В роли представителя Манибел, мы нацелены на то, чтобы помочь каждому клиенту преодолеть свои денежные трудности, предложив ясные и выгодные условия займа. Доверьтесь нашей компании, и мы поможем вам в решении ваших финансовых проблем.
Написание дипломных работ https://diplompishem.ru/, курсовых и рефератов от лучших авторов. Заказать студенческую работу с антиплагиатом и уникализацией.
В сегодняшнем обществе финансовые сложности не редкость, и каждый человек может оказаться в ситуации, когда потребуется дополнительная финансовая помощь. На сайте [url=https://moneybel.ru/]деньги в долг в Минске[/url] помогут преодолеть эти трудности, предоставляя простой и удобный способ получения кредита. Мы понимаем, что времени на решение проблемы не хватает, поэтому мы обрабатываем заявки оперативно и эффективно. В качестве представителя Манибел, наша цель – помочь каждому клиенту преодолеть финансовые затруднения, предоставив прозрачные и выгодные условия кредитования. Доверьтесь нам, и мы поможем вам в решении ваших финансовых проблем.
В настоящей эпохе финансовые трудности не редкость, и каждый человек может оказаться в ситуации, когда необходима дополнительная финансовая поддержка. На сайте [url=https://moneybel.ru/]взять деньги в долг[/url] помогут преодолеть эти трудности, принося простой и удобный способ получения кредита. Мы понимаем, что времени на решение проблемы не хватает, поэтому мы обрабатываем заявки оперативно и эффективно. В качестве представителя Манибел, наша цель – помочь каждому клиенту преодолеть финансовые затруднения, предложив ясные и выгодные условия займа. Доверьтесь нашей компании, и мы решим ваши финансовые проблемы.
обучение на барбера [url=https://www.burberi.ru]https://www.burberi.ru[/url] .
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]транспортерная лента на Bellenty[/url] помогут консультанты интернет-магазина. Благодаря нашему опыту и компетентности мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам качества и эффективности. Мы производим индивидуальные решения, учитывая особые потребности наших клиентов. Независимо от величины, материала или технических характеристик, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]купить ленты транспортерные на Bellenty.by[/url] помогут консультанты интернет-магазина. Благодаря нашему практике и профессионализму мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам стандартам качества и эффективности. Мы изготавливаем индивидуальные решения, учитывая уникальные потребности наших заказчиков. Независимо от размера, материала или технических характеристик, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]заказать транспортерные ленты на Bellenty.by[/url] помогут консультанты магазина. Благодаря нашему практике и профессионализму мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам стандартам качества и эффективности. Мы производим индивидуальные решения, учитывая особые потребности наших клиентов. Независимо от размера, материала или технических характеристик, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]транспортерные ленты[/url] помогут консультанты интернет-магазина. Благодаря нашему практике и профессионализму мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам стандартам качества и эффективности. Мы изготавливаем индивидуальные решения, учитывая особые потребности наших заказчиков. Независимо от величины, материала или технических характеристик, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
На сайте https://greateastsiberia.ru/ почитайте поучительную, интересную и увлекательную информацию, которая касается здорового образа жизни. Имеются ценные, важные рекомендации относительно правильного питания, занятий спортом и других моментов. Есть информация о том, как сохранить суставы, о народных методах. Имеются рекомендации о том, как сохранить здоровье самых маленьких. Вы узнаете о том, как правильно бегать для получения необходимого эффекта. С вами поделятся своими рецептами для того, чтобы достичь гармонии души.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]конвейерная лента в Минске[/url] помогут консультанты магазина. Благодаря нашему практике и профессионализму мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам качества и эффективности. Мы изготавливаем индивидуальные решения, учитывая особые потребности наших клиентов. Независимо от величины, материала или технических характеристик, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]купить конвейерную ленту на Bellenty[/url] помогут консультанты магазина. Благодаря нашему практике и профессионализму мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам стандартам качества и эффективности. Мы производим индивидуальные решения, учитывая уникальные потребности наших заказчиков. Независимо от размера, материала или спецификаций, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
На сайте https://historical-notebook.com/ ознакомьтесь с любопытными и интересными данными, касающимися истории возникновения земли. Есть информация и про древнюю цивилизацию. Ознакомьтесь с материалами о мифическом правителе Горе. Имеются и свежие записи, которые точно будут вам любопытны. Почитайте заметки о полководце Александре Суворове. Есть информация и о первом президенте Америки. Вся она является актуальной и достоверной. Много данных о ключевых персонах, сыгравших огромную роль в истории.
SEO продвижение и раскрутка сайтов с гарантией https://seomayker.ru/. Услуги по продвижению сайта в поисковых системах. Видимость вашего сайта в поисковых системах повысится и вы привлечете больше качественных посетителей без необходимости платить за рекламу.
Стоматология в Первоуральске ДентаЛюкс https://dlux.dental/ это обширный перечень стоматологических услуг, таких как: лечение зубов и кариеса, имплантация зубов, протезирование зубов, детская стоматология, в том числе лечение молочных зубов и многое другое. Удобное месторасположение, приятные цены, профессиональные специалисты.
[url=https://valtrexv.com/]valtrex 500 mg tablet[/url]
Поисковое SEO продвижение сайтов в Москве https://seosferaya.ru/, стоимость тарифов на быструю и недорогую раскрутку сайта в ТОП-10. Оптимизация сайтов в поисковых системах Яндекс и Google. Быстро раскручиваем сайты за счет опыта. Тарифы и цены, кейсы и результаты наших работ в Москве и других крупных городах РФ
Чим корисні тактичні рюкзаки
Відмінності від звичайних
купити рюкзак тактичний [url=https://ryukzakivijskovibpjgl.kiev.ua/]купити рюкзак тактичний[/url] .
кортинефф купить польский
Комплексное продвижение сайтов в Москве https://seosferaya.ru/ под ключ. SEO раскрутка сайта в топ Яндекс и Google от профессионалов, первые результаты уже через месяц. Комплексное продвижение сайтов с гарантией.
best cs live gambling site
скачать фильм викинг
онлайн казино джет казино
Я заказывал аквариум на этом сайте. Аквариум травник на заказ
[url=https://isynthroid.com/]generic synthroid cost[/url]
я уже смотрел обзор здесь https://my-obzor.com/ перед тем, как сделать заказ. Не сказать, что все отзывы были 100% положительные, там уже упоминались основные минусы и плюсы
мебель под заказ
На сайте https://iz-nerzhaveyki.ru/ ознакомьтесь с трубами из нержавейки, гибкой подводкой, шаровыми кранами, фитингами, фланцевыми кранами, обратными клапанами, арматурой и многим другим. В разделе изучите технические характеристики. Они бывают самых разных модификаций, диаметра, а также размеров, что позволит выбрать решение, основываясь на свои предпочтения, требования, цели покупки. Для большей наглядности имеются фотографии всех товаров, что позволит быстро сориентироваться в выборе.
Go to the website https://binomo-app.online/ and you will learn everything about Binomo – the binary options platform, including detailed manuals for depositing and withdrawing funds, logging in and registering on the platform, for profitable trading in more than 100 instruments. You can also download the Binomo application for all devices.
https://zmiev.org.ua/
https://petroyalportrait.com/
USS.EU.COM is excited to announce its platform, offering a unique opportunity for authors to publish guest posts for free, become recognized as top writers, and earn alongside us. Additionally, we provide a free site catalog, positioning ourselves as the best alternative to DMOZ.
Key Features of USS.EU.COM:
Free Guest Posting
Earnings for Top Authors
Comprehensive Site Catalog
На сайте https://wedding42.ru/ почитайте информацию, которая касается того, как правильно, быстро и без хлопот организовать свадьбу мечты. Имеются материалы о том, как красиво и изысканно оформить помещение, какое платье лучше всего подобрать. Опубликована информация об организации СПА для жениха и невесты. Обязательно ознакомьтесь с рубриками, посвященными гостям на свадьбе, обычаям, свадебному путешествию, подаркам. Возможно, вам понравятся свадебные платья со шлейфом. Перед вами несколько вариантов, среди которых вы подберете что-то для себя.
Pingback: venlafaxine and alcohol reviews
https://petroyalportrait.com/
Pingback: tamsulosin hydrochloride action
https://nullkong.com/
Stumbled upon a unique article, I suggest you take a look https://www.federalbaseball.com/users/cchatruletka
[url=http://medicinesaf.online/]good pharmacy[/url]
На сайте https://solvki-land.ru закажите увлекательную, познавательную экскурсию, рассчитанную на туристов различного возраста «Соловецкий феномен». Вы ознакомитесь с 10 экскурсиями, которые вы не сможете заказать ни в каком другом месте. Они длятся в течение 6 или 8 дней. На портале ознакомьтесь с природой Соловков, а в рамках экскурсий вы увидите Соловецкий сад, который представляет собой особую достопримечательность. На сайте вы увидите всю красоту острова, включая ландшафты, природу и многое другое.
Привет любители поэзии!
Всем, кто проникается стихами, поэмами и собственным творчеством, Мay Poems предлагает лучшие произведения великих мировых поэтов и молодых талантов поэзии!
Я сам только начинающий поэт, и этот портал помог мне приобрести уверенность и писать более увлекательно и искренне.
Лучший способ погрузиться в мир поэзии и получать удовольствие от нее – читать стихи и творить свои собственные, а также обмениваться ими в сообществах и обсуждать с друзьями.
Поэзия действительно захватывает ум и душу, и вдохновляет на личный рост. Я рекомендую вам проводить время с пользой, занимаясь тем, что вас по-настоящему интересует!
Все самое лучшее на сайте https://may-poems.ru/
Стихи Александра Блока
Стихотворения Некрасова
Поэзия Александра Пушкина
Стихи Федора Достоевского
Удачи!
Pingback: synthroid lipitor
CRUDERRA https://cruderra.com/ – fast TechDocs for engineering teams. DiagramGPT and Architecture as code. Create docs to speed up development, onboarding & legacy code modernization.
Pingback: voltaren gel okay with coumadin
я уже смотрел обзор здесь https://my-obzor.com/ перед тем, как сделать заказ. Не сказать, что все отзывы были 100% положительные, там уже упоминались основные минусы и плюсы.
[url=http://bmtadalafil.online/]can you buy tadalafil online[/url]
Pingback: zinc and spironolactone reddit
На сайте https://fordrazbor.ru/ уточните то, где проходит авторазбор Ford. Перед вами топ сервисов, где проводится авторазбор. Здесь продают как б/у запчасти, так и те, что использовались совсем немного. От того, сколько детали находились в эксплуатации, зависит их итоговая стоимость. У некоторых компаний имеется онлайн-каталог, где можно посмотреть те товары, которые имеются в наличии, а также ознакомиться с ценами. А есть и такие авторазборы, где сразу же установят необходимую запчасть.
Opened up interesting material – I recommend sharing this discovery http://censornet.ru/otkroy-dlya-sebya-novyie-gorizontyi-znakomstva-s-devushkami-onlayn/
На сайте https://t-audio.com/ оформите заявку для того, чтобы заказать такую важную услугу, как техническое обеспечение мероприятий. Компания находится на рынке в течение длительного времени, а потому выучила все потребности клиентов. В ней коллектив с огромным опытом, внушительный парк светового, звукового, а также сценического оборудования. Действуют привлекательные расценки, система скидок для постоянных клиентов. Для того чтобы ознакомиться с примерами работ, зайдите в портфолио.
Зайдите на сайт ARSI Group https://arsi.kz/ которая является оптовым поставщиком комплектующих для металлопластиковых окон, дверей и светопроводящих конструкций, а также фурнитуры для ПВХ окон и дверей REZE, ПВХ-профиля WDS, монтажной пены REALIST и подоконников премиум класса DANKE и многое другое. Подробнее на сайте.
mebelminsk
На сайте http://profotonsk.ru профессиональный фотограф предлагает свою услугу по специализации – интерьерная фотосессия. Большой опыт работы и множество рекламных фотосессий позволяет фотографу работать как с частными риэлторами, так и с агентствами недвижимости. В своей работе он применяет камеру с полноформатным сенсором и широкоугольный объектив, это обеспечивает отличное качество изображения, у вас есть возможность в этом лично убедиться. Подробнее посмотреть услуги фотографа, отзывы, а также его портфолио, вы можете на портале.
В настоящей эпохе финансовые трудности встречаются часто, и каждый из нас может оказаться в ситуации, когда потребуется дополнительная финансовая помощь. На сайте [url=https://moneybel.ru/]МаниБел[/url] помогут преодолеть эти трудности, предоставляя простой и удобный способ займа. Мы понимаем, что время играет ключевую роль, поэтому мы обрабатываем заявки оперативно и эффективно. В роли представителя Манибел, наша цель – помочь каждому клиенту преодолеть финансовые затруднения, предложив ясные и выгодные условия займа. Доверьтесь нашей компании, и мы решим ваши финансовые проблемы.
В настоящей эпохе финансовые сложности не редкость, и каждый человек может оказаться в ситуации, когда потребуется дополнительная финансовая помощь. На сайте [url=https://moneybel.ru/]займы онлайн[/url] помогут преодолеть эти трудности, принося простой и удобный способ займа. Мы понимаем, что времени на решение проблемы не хватает, поэтому наш процесс обработки заявок максимально быстр и эффективен. В качестве представителя Манибел, мы нацелены на то, чтобы помочь каждому клиенту преодолеть свои денежные трудности, предоставив прозрачные и выгодные условия кредитования. Доверьтесь нашей компании, и мы решим ваши финансовые проблемы.
В сегодняшнем обществе финансовые сложности встречаются часто, и каждый человек может оказаться в ситуации, когда необходима дополнительная финансовая поддержка. На сайте [url=https://moneybel.ru/]взять деньги в долг[/url] помогут решить эти проблемы, принося простой и лёгкий метод займа. Мы понимаем, что времени на решение проблемы не хватает, поэтому наш процесс обработки заявок максимально быстр и эффективен. В роли представителя Манибел, мы нацелены на то, чтобы помочь каждому клиенту преодолеть свои денежные трудности, предложив ясные и выгодные условия займа. Доверьтесь нашей компании, и мы решим ваши финансовые проблемы.
В сегодняшнем обществе финансовые трудности встречаются часто, и каждый человек может оказаться в ситуации, когда необходима дополнительная финансовая поддержка. На сайте [url=https://moneybel.ru/]займ денег[/url] помогут решить эти проблемы, предоставляя простой и удобный способ займа. Мы осознаём, что времени на решение проблемы не хватает, поэтому мы обрабатываем заявки оперативно и эффективно. В роли представителя Манибел, мы нацелены на то, чтобы помочь каждому клиенту преодолеть свои денежные трудности, предоставив прозрачные и выгодные условия кредитования. Доверьтесь нашей компании, и мы поможем вам в решении ваших финансовых проблем.
В сегодняшнем обществе финансовые сложности не редкость, и каждый человек может оказаться в ситуации, когда потребуется дополнительная финансовая помощь. На сайте [url=https://moneybel.ru/]получить займ в Минске[/url] помогут решить эти проблемы, принося простой и лёгкий метод займа. Мы осознаём, что времени на решение проблемы не хватает, поэтому наш процесс обработки заявок максимально быстр и эффективен. В роли представителя Манибел, наша цель – помочь каждому клиенту преодолеть финансовые затруднения, предложив ясные и выгодные условия займа. Доверьтесь нам, и мы решим ваши финансовые проблемы.
В сегодняшнем обществе финансовые трудности встречаются часто, и каждый человек может оказаться в ситуации, когда потребуется дополнительная финансовая помощь. На сайте [url=https://moneybel.ru/]займ в беларуси[/url] помогут преодолеть эти трудности, предоставляя простой и удобный способ займа. Мы осознаём, что время играет ключевую роль, поэтому мы обрабатываем заявки оперативно и эффективно. В роли представителя Манибел, мы нацелены на то, чтобы помочь каждому клиенту преодолеть свои денежные трудности, предложив ясные и выгодные условия займа. Доверьтесь нашей компании, и мы решим ваши финансовые проблемы.
В сегодняшнем обществе финансовые сложности не редкость, и каждый человек может оказаться в ситуации, когда необходима дополнительная финансовая поддержка. На сайте [url=https://moneybel.ru/]взять деньги в долг[/url] помогут преодолеть эти трудности, предоставляя простой и удобный способ получения кредита. Мы осознаём, что время играет ключевую роль, поэтому мы обрабатываем заявки оперативно и эффективно. В роли представителя Манибел, мы нацелены на то, чтобы помочь каждому клиенту преодолеть свои денежные трудности, предоставив прозрачные и выгодные условия кредитования. Доверьтесь нам, и мы поможем вам в решении ваших финансовых проблем.
В настоящей эпохе финансовые трудности встречаются часто, и каждый из нас может оказаться в ситуации, когда потребуется дополнительная финансовая помощь. На сайте [url=https://moneybel.ru/]займ в Минске[/url] помогут решить эти проблемы, предоставляя простой и лёгкий метод займа. Мы понимаем, что время играет ключевую роль, поэтому наш процесс обработки заявок максимально быстр и эффективен. В роли представителя Манибел, мы нацелены на то, чтобы помочь каждому клиенту преодолеть свои денежные трудности, предложив ясные и выгодные условия займа. Доверьтесь нам, и мы решим ваши финансовые проблемы.
https://seostrategia.ru/
В настоящей экономической обстановке, где неожиданные финансовые обязательства могут возникнуть в любое время, поиск надежного и удобного источника финансовой помощи становится все более критическим. Предложение [url=https://zaim-minsk.ru/]деньги в долг в Минске[/url] предлагает возможность рассчитывать на быстрое и беззаботное получение необходимых средств без лишних трудностей. Мы ценим ваше время и обеспечиваем оперативную обработку всех запросов. В качестве вашего финансового партнера, наша главная задача состоит в том, чтобы помочь вам преодолеть текущие финансовые препятствия, предоставляя ясные и выгодные условия кредитования. Доверьтесь нам в вашем финансовом путешествии, и мы обеспечим вас надежной поддержкой на каждом этапе.
В настоящей экономической обстановке, где неожиданные финансовые обязательства могут возникнуть в любое время, поиск надежного и удобного источника финансовой помощи становится все более критическим. Предложение [url=https://zaim-minsk.ru/]деньги в долг[/url] предлагает возможность рассчитывать на быстрое и беззаботное получение необходимых средств без лишних трудностей. Мы ценим ваше время и обеспечиваем оперативную обработку всех запросов. В качестве вашего финансового партнера, наша главная задача состоит в том, чтобы помочь вам преодолеть текущие финансовые препятствия, предоставляя ясные и выгодные условия кредитования. Доверьтесь нам в вашем финансовом путешествии, и мы обеспечим вас надежной поддержкой на каждом этапе.
груз из казахстана в россию https://perevozka-iz-kz.ru/
PBN sites
We will generate a web of self-owned blog network sites!
Advantages of our self-owned blog network:
We execute everything so GOOGLE DOES NOT realize that this A self-owned blog network!!!
1- We buy domains from separate registrars
2- The main site is hosted on a virtual private server (Virtual Private Server is fast hosting)
3- The rest of the sites are on various hostings
4- We designate a individual Google ID to each site with verification in Google Search Console.
5- We develop websites on WordPress, we don’t use plugins with aided by which Trojans penetrate and through which pages on your websites are generated.
6- We never repeat templates and use only individual text and pictures
We do not work with website design; the client, if wished, can then edit the websites to suit his wishes
В настоящей экономической обстановке, где неожиданные финансовые обязательства могут возникнуть в любое время, поиск надежного и удобного источника финансовой помощи становится все более критическим. Предложение [url=https://zaim-minsk.ru/]zaim-minsk.ru[/url] предлагает возможность рассчитывать на быстрое и беззаботное получение необходимых средств без лишних трудностей. Мы ценим ваше время и обеспечиваем оперативную обработку всех запросов. В качестве вашего финансового партнера, наша главная задача состоит в том, чтобы помочь вам преодолеть текущие финансовые препятствия, предоставляя ясные и выгодные условия кредитования. Доверьтесь нам в вашем финансовом путешествии, и мы обеспечим вас надежной поддержкой на каждом этапе.
В настоящей экономической обстановке, где неожиданные финансовые обязательства могут возникнуть в любое время, поиск надежного и удобного источника финансовой помощи становится все более критическим. Предложение [url=https://zaim-minsk.ru/]займы онлайн[/url] предлагает возможность рассчитывать на быстрое и беззаботное получение необходимых средств без лишних трудностей. Мы ценим ваше время и обеспечиваем оперативную обработку всех запросов. В качестве вашего финансового партнера, наша главная задача состоит в том, чтобы помочь вам преодолеть текущие финансовые препятствия, предоставляя ясные и выгодные условия кредитования. Доверьтесь нам в вашем финансовом путешествии, и мы обеспечим вас надежной поддержкой на каждом этапе.
В настоящей экономической обстановке, где неожиданные финансовые обязательства могут возникнуть в любое время, поиск надежного и удобного источника финансовой помощи становится все более критическим. Предложение [url=https://zaim-minsk.ru/]займ онлайн[/url] предлагает возможность рассчитывать на быстрое и беззаботное получение необходимых средств без лишних трудностей. Мы ценим ваше время и обеспечиваем оперативную обработку всех запросов. В качестве вашего финансового партнера, наша главная задача состоит в том, чтобы помочь вам преодолеть текущие финансовые препятствия, предоставляя ясные и выгодные условия кредитования. Доверьтесь нам в вашем финансовом путешествии, и мы обеспечим вас надежной поддержкой на каждом этапе.
Скачать Winline апп
В настоящей экономической обстановке, где неожиданные финансовые обязательства могут возникнуть в любое время, поиск надежного и удобного источника финансовой помощи становится все более критическим. Предложение [url=https://zaim-minsk.ru/]взять деньги[/url] предлагает возможность рассчитывать на быстрое и беззаботное получение необходимых средств без лишних трудностей. Мы ценим ваше время и обеспечиваем оперативную обработку всех запросов. В качестве вашего финансового партнера, наша главная задача состоит в том, чтобы помочь вам преодолеть текущие финансовые препятствия, предоставляя ясные и выгодные условия кредитования. Доверьтесь нам в вашем финансовом путешествии, и мы обеспечим вас надежной поддержкой на каждом этапе.
В настоящей экономической обстановке, где неожиданные финансовые обязательства могут возникнуть в любое время, поиск надежного и удобного источника финансовой помощи становится все более критическим. Предложение [url=https://zaim-minsk.ru/]деньги в долг[/url] предлагает возможность рассчитывать на быстрое и беззаботное получение необходимых средств без лишних трудностей. Мы ценим ваше время и обеспечиваем оперативную обработку всех запросов. В качестве вашего финансового партнера, наша главная задача состоит в том, чтобы помочь вам преодолеть текущие финансовые препятствия, предоставляя ясные и выгодные условия кредитования. Доверьтесь нам в вашем финансовом путешествии, и мы обеспечим вас надежной поддержкой на каждом этапе.
Зайдите на сайт FishX https://fishx.org/ и вы найдете все о рыбалке, cнастях, прикормке, приманках, способах ловле, а также отчёты о рыбной ловле. Ознакомьтесь с видами рыб и на что они ловятся, их места обитания. Сайт будет полезен как начинающим рыбакам, так и профи с опытом рыбной ловли.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]конвейерная лента на Bellenty[/url] помогут консультанты магазина. Благодаря нашему опыту и компетентности мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам качества и эффективности. Мы разрабатываем индивидуальные решения, учитывая уникальные потребности наших заказчиков. Независимо от величины, материала или технических характеристик, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]ленты транспортерные[/url] помогут консультанты интернет-магазина. Благодаря нашему практике и профессионализму мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам стандартам качества и эффективности. Мы производим индивидуальные решения, учитывая особые потребности наших заказчиков. Независимо от величины, состава или технических характеристик, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]цена лент конвейерных на Bellenty[/url] помогут консультанты магазина. Благодаря нашему опыту и профессионализму мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам стандартам качества и эффективности. Мы разрабатываем индивидуальные решения, учитывая уникальные потребности наших клиентов. Независимо от размера, состава или технических характеристик, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
MIGRANT SUPPORT – компания, успешно предоставляющая услуги по оформлению транспортной лицензии в Польше. Мы сконцентрированы на действенном решении задач своих клиентов. Всегда рады детально ответить на все ваши вопросы и помочь вам. Ждем ваши обращения с нетерпением. https://vpolshe.com/transportnaya-litsenziya-v-polshe/ – сайт, где представлена полезная информация о транспортной лицензии в Польше. Гарантируем вам доступную стоимость. Вы можете получить профессиональную консультацию уже сейчас. Пишите нам и звоните!
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]цена лент конвейерных[/url] помогут консультанты интернет-магазина. Благодаря нашему практике и профессионализму мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам стандартам качества и эффективности. Мы производим индивидуальные решения, учитывая уникальные потребности наших заказчиков. Независимо от величины, состава или спецификаций, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]транспортерная лента[/url] помогут консультанты интернет-магазина. Благодаря нашему опыту и компетентности мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам качества и эффективности. Мы изготавливаем индивидуальные решения, учитывая особые потребности наших клиентов. Независимо от величины, материала или технических характеристик, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]заказать ленты конвейерные на Bellenty.by[/url] помогут консультанты магазина. Благодаря нашему опыту и компетентности мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам стандартам качества и эффективности. Мы изготавливаем индивидуальные решения, учитывая особые потребности наших клиентов. Независимо от величины, материала или спецификаций, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]стоимость лент транспортерных в Минске[/url] помогут консультанты интернет-магазина. Благодаря нашему опыту и профессионализму мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам стандартам качества и эффективности. Мы разрабатываем индивидуальные решения, учитывая особые потребности наших клиентов. Независимо от размера, состава или спецификаций, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]оформить транспортерную ленту в Минске[/url] помогут консультанты магазина. Благодаря нашему практике и профессионализму мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам качества и эффективности. Мы изготавливаем индивидуальные решения, учитывая уникальные потребности наших заказчиков. Независимо от величины, состава или спецификаций, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
В настоящее время наши дни могут содержать внезапные издержки и финансовые вызовы, и в такие моменты каждый ищет помощь. На сайте [url=https://brokers-group.ru/]brokers-group.ru[/url] вам помогут без лишних сложностей. В своей роли агента я стремлюсь распространить эту информацию, чтобы помочь тем, кто столкнулся с временными трудностями. Наша система обеспечивает честные условия и быструю обработку заявок, чтобы каждый мог решить свои денежные проблемы быстро и без лишних хлопот.
[url=http://isynthroid.com/]price for 125 mcg synthroid[/url]
На сайте https://vitaminium.shop/ закажите витамины, БАДы, минеральные комплексы, которые идеально подходят для всей семьи. Заказы принимаются круглосуточно. Их оформляют через корзину и в любое время. На всю продукцию имеются сертификаты. А если остались вопросы, то задайте их менеджерам в техподдержку. Оплата товаров является абсолютно безопасной, конфиденциальной. Имеются витамины, которые созданы специально для укрепления женского здоровья. Вся продукция реализуется по привлекательным расценкам и с оперативной доставкой.
В настоящее время наши дни могут содержать неожиданные расходы и экономические трудности, и в такие моменты каждый ищет надежную поддержку. На сайте [url=https://brokers-group.ru/]деньги в долг в Минске срочно[/url] вам помогут без избыточной бюрократии. В своей роли представителя я стремлюсь распространить эту информацию, чтобы помочь людям в сложной ситуации. Наша платформа обеспечивает прозрачные условия и быструю обработку заявок, чтобы каждый мог разрешить свои финансовые вопросы быстро и без лишних хлопот.
В настоящее время наши дни могут содержать неожиданные расходы и экономические трудности, и в такие моменты каждый ищет помощь. На сайте [url=https://brokers-group.ru/]получить деньги в долг в Минске[/url] вам помогут без лишних сложностей. В своей роли представителя я стремлюсь поделиться этими сведениями, чтобы помочь тем, кто столкнулся с временными трудностями. Наша система обеспечивает честные условия и эффективное рассмотрение запросов, чтобы каждый мог разрешить свои финансовые вопросы оперативно и без лишних затрат времени.
В настоящее время наши дни могут содержать внезапные издержки и экономические трудности, и в такие моменты каждый ищет надежную поддержку. На сайте [url=https://brokers-group.ru/]займ в Минске[/url] вам помогут без лишних сложностей. В своей роли представителя я стремлюсь распространить эту информацию, чтобы помочь тем, кто столкнулся с временными трудностями. Наша платформа обеспечивает честные условия и быструю обработку заявок, чтобы каждый мог разрешить свои финансовые вопросы быстро и без лишних хлопот.
В настоящее время наши дни могут содержать неожиданные расходы и финансовые вызовы, и в такие моменты каждый ищет помощь. На сайте [url=https://brokers-group.ru/]займ в Минске[/url] вам помогут без избыточной бюрократии. В своей роли агента я стремлюсь поделиться этими сведениями, чтобы помочь тем, кто столкнулся с временными трудностями. Наша платформа обеспечивает прозрачные условия и быструю обработку заявок, чтобы каждый мог решить свои денежные проблемы оперативно и без лишних затрат времени.
Pingback: stromectol price in india
В настоящее время наши дни могут содержать неожиданные расходы и финансовые вызовы, и в такие моменты каждый ищет помощь. На сайте [url=https://brokers-group.ru/]деньги в долг[/url] вам помогут без избыточной бюрократии. В своей роли представителя я стремлюсь поделиться этими сведениями, чтобы помочь тем, кто столкнулся с временными трудностями. Наша система обеспечивает прозрачные условия и быструю обработку заявок, чтобы каждый мог решить свои денежные проблемы оперативно и без лишних затрат времени.
стенд ролл ап купить [url=https://www.rollap.ru/]https://www.rollap.ru/[/url] .
В настоящее время наши дни могут содержать внезапные издержки и финансовые вызовы, и в такие моменты каждый ищет помощь. На сайте [url=https://brokers-group.ru/]получить деньги в долг в Минске[/url] вам помогут без избыточной бюрократии. В своей роли представителя я стремлюсь распространить эту информацию, чтобы помочь людям в сложной ситуации. Наша платформа обеспечивает прозрачные условия и быструю обработку заявок, чтобы каждый мог разрешить свои финансовые вопросы оперативно и без лишних затрат времени.
В настоящее время наши дни могут содержать внезапные издержки и экономические трудности, и в такие моменты каждый ищет надежную поддержку. На сайте [url=https://brokers-group.ru/]взять деньги в долг[/url] вам помогут без лишних сложностей. В своей роли агента я стремлюсь распространить эту информацию, чтобы помочь тем, кто столкнулся с временными трудностями. Наша платформа обеспечивает прозрачные условия и быструю обработку заявок, чтобы каждый мог решить свои денежные проблемы оперативно и без лишних затрат времени.
[url=https://bestprednisone.online/]prednisone order online uk[/url]
В наше время многие из нас сталкиваются с внезапными затратами и экономическими вызовами, и в такие моменты важно иметь возможность взять кредит. На сайте [url=https://dengizaimy.ru/]займ в РБ[/url] вам помогут с минимальными усилиями. В качестве официального представителя Брокерс Групп, я с удовольствием делиться этой важной информацией, чтобы помочь каждому, кто нуждается в финансовой поддержке. Наша платформа гарантирует честные условия и оперативное рассмотрение запросов, чтобы каждый мог разрешить свои денежные проблемы с минимальными временными затратами.
В наше время многие из нас сталкиваются с непредвиденными расходами и экономическими вызовами, и в такие моменты важно иметь возможность взять кредит. На сайте [url=https://dengizaimy.ru/]деньги в долг на карту[/url] вам помогут быстро и удобно. В качестве официального представителя Брокерс Групп, я рад делиться этой важной информацией, чтобы помочь каждому, кто нуждается в финансовой поддержке. Наша система гарантирует прозрачные условия и оперативное рассмотрение запросов, чтобы каждый мог решить свои финансовые вопросы с минимальными временными затратами.
В наше время многие из нас сталкиваются с непредвиденными расходами и денежными трудностями, и в такие моменты важно иметь доступ к дополнительным средствам. На сайте [url=https://dengizaimy.ru/]деньги в долг в Минске[/url] вам помогут с минимальными усилиями. В качестве официального представителя Брокерс Групп, я рад делиться этой важной информацией, чтобы помочь каждому, кто нуждается в финансовой поддержке. Наша платформа гарантирует прозрачные условия и оперативное рассмотрение запросов, чтобы каждый мог разрешить свои денежные проблемы быстро и эффективно.
В наше время многие из нас сталкиваются с непредвиденными расходами и денежными трудностями, и в такие моменты важно иметь доступ к дополнительным средствам. На сайте [url=https://dengizaimy.ru/]займ в Минске[/url] вам помогут с минимальными усилиями. В качестве официального представителя Брокерс Групп, я рад делиться этой важной информацией, чтобы помочь людям, кто столкнулся с финансовыми трудностями. Наша система гарантирует честные условия и оперативное рассмотрение запросов, чтобы каждый мог решить свои финансовые вопросы с минимальными временными затратами.
В наше время многие из нас сталкиваются с непредвиденными расходами и экономическими вызовами, и в такие моменты важно иметь возможность взять кредит. На сайте [url=https://dengizaimy.ru/]деньги в кредит[/url] вам помогут быстро и удобно. В качестве представителя Брокерс Групп, я с удовольствием делиться этой важной информацией, чтобы помочь людям, кто столкнулся с финансовыми трудностями. Наша платформа гарантирует прозрачные условия и оперативное рассмотрение запросов, чтобы каждый мог разрешить свои денежные проблемы с минимальными временными затратами.
В наше время многие из нас сталкиваются с внезапными затратами и денежными трудностями, и в такие моменты важно иметь доступ к дополнительным средствам. На сайте [url=https://dengizaimy.ru/]займы онлайн на карту[/url] вам помогут с минимальными усилиями. В качестве представителя Брокерс Групп, я рад делиться этой важной информацией, чтобы помочь людям, кто столкнулся с финансовыми трудностями. Наша система гарантирует честные условия и оперативное рассмотрение запросов, чтобы каждый мог разрешить свои денежные проблемы быстро и эффективно.
[url=https://happyfamilystorerx.online/]discount pharmacy online[/url]
Ищите повышение квалификации и переподготовку логопедов? Тогда вам необходимо посетить https://logopraktika.ru/ где вы сможете ознакомиться с программами повышения квалификации логопедов по всей России в формате дистанционного обучения. Подробнее на сайте.
Pingback: tizanidine drug interactions
В наше время многие из нас сталкиваются с непредвиденными расходами и экономическими вызовами, и в такие моменты важно иметь доступ к дополнительным средствам. На сайте [url=https://dengizaimy.ru/]деньги в долг в Минске[/url] вам помогут быстро и удобно. В качестве официального представителя Брокерс Групп, я с удовольствием делиться этой информацией, чтобы помочь людям, кто столкнулся с финансовыми трудностями. Наша платформа гарантирует прозрачные условия и быструю обработку заявок, чтобы каждый мог разрешить свои денежные проблемы с минимальными временными затратами.
На сайте https://www.techno-svyaz.ru/ разместите заказ на то, чтобы заказать печатные платы напрямую от производителя. Специально для вас действует оперативное производство. Есть возможность воспользоваться экспресс услугой. Вас ожидают короткие сроки, а также привлекательные расценки. Возможно заказать средние и мелкие серии печатных плат. Вся продукция отличается высоким качеством, на нее распространяются гарантии. Вы сможете заказать маркирование деталей. При необходимости воспользуйтесь консультацией.
В наше время многие из нас сталкиваются с внезапными затратами и экономическими вызовами, и в такие моменты важно иметь доступ к дополнительным средствам. На сайте [url=https://dengizaimy.ru/]получить деньги в долг в Минске[/url] вам помогут быстро и удобно. В качестве официального представителя Брокерс Групп, я с удовольствием делиться этой информацией, чтобы помочь людям, кто столкнулся с финансовыми трудностями. Наша система гарантирует честные условия и быструю обработку заявок, чтобы каждый мог решить свои финансовые вопросы с минимальными временными затратами.
В наше время многие из нас сталкиваются с непредвиденными расходами и денежными трудностями, и в такие моменты важно иметь возможность взять кредит. На сайте [url=https://dengizaimy.ru/]займ денег[/url] вам помогут с минимальными усилиями. В качестве официального представителя Брокерс Групп, я рад делиться этой важной информацией, чтобы помочь людям, кто нуждается в финансовой поддержке. Наша платформа гарантирует прозрачные условия и быструю обработку заявок, чтобы каждый мог разрешить свои денежные проблемы быстро и эффективно.
В наше время многие из нас сталкиваются с внезапными затратами и денежными трудностями, и в такие моменты важно иметь доступ к дополнительным средствам. На сайте [url=https://dengizaimy.ru/]dengizaimy.ru[/url] вам помогут с минимальными усилиями. В качестве официального представителя Брокерс Групп, я рад делиться этой информацией, чтобы помочь каждому, кто столкнулся с финансовыми трудностями. Наша система гарантирует честные условия и оперативное рассмотрение запросов, чтобы каждый мог решить свои финансовые вопросы быстро и эффективно.
В наше время многие из нас сталкиваются с внезапными затратами и денежными трудностями, и в такие моменты важно иметь доступ к дополнительным средствам. На сайте [url=https://dengizaimy.ru/]взять деньги в долг в Минске[/url] вам помогут с минимальными усилиями. В качестве представителя Брокерс Групп, я рад делиться этой информацией, чтобы помочь людям, кто столкнулся с финансовыми трудностями. Наша система гарантирует честные условия и оперативное рассмотрение запросов, чтобы каждый мог разрешить свои денежные проблемы с минимальными временными затратами.
В наше время многие из нас сталкиваются с внезапными затратами и экономическими вызовами, и в такие моменты важно иметь доступ к дополнительным средствам. На сайте [url=https://dengizaimy.ru/]займ денег минск[/url] вам помогут с минимальными усилиями. В качестве представителя Брокерс Групп, я рад делиться этой важной информацией, чтобы помочь каждому, кто столкнулся с финансовыми трудностями. Наша платформа гарантирует честные условия и быструю обработку заявок, чтобы каждый мог решить свои финансовые вопросы быстро и эффективно.
В наше время многие из нас сталкиваются с внезапными затратами и экономическими вызовами, и в такие моменты важно иметь доступ к дополнительным средствам. На сайте [url=https://dengizaimy.ru/]деньги в долг[/url] вам помогут с минимальными усилиями. В качестве представителя Брокерс Групп, я рад делиться этой информацией, чтобы помочь каждому, кто нуждается в финансовой поддержке. Наша платформа гарантирует честные условия и оперативное рассмотрение запросов, чтобы каждый мог решить свои финансовые вопросы быстро и эффективно.
В наше время многие из нас сталкиваются с внезапными затратами и денежными трудностями, и в такие моменты важно иметь доступ к дополнительным средствам. На сайте [url=https://dengizaimy.ru/]ДеньгиЗаймы[/url] вам помогут быстро и удобно. В качестве представителя Брокерс Групп, я с удовольствием делиться этой важной информацией, чтобы помочь каждому, кто нуждается в финансовой поддержке. Наша система гарантирует прозрачные условия и быструю обработку заявок, чтобы каждый мог решить свои финансовые вопросы с минимальными временными затратами.
Pingback: dapagliflozin vs sitagliptin
В сегодняшнем обществе финансовые трудности не редкость, и каждый человек может оказаться в ситуации, когда необходима дополнительная финансовая поддержка. На сайте [url=https://moneybel.ru/]займ на карту без отказа[/url] помогут решить эти проблемы, принося простой и лёгкий метод получения кредита. Мы осознаём, что время играет ключевую роль, поэтому наш процесс обработки заявок максимально быстр и эффективен. В роли представителя Манибел, мы нацелены на то, чтобы помочь каждому клиенту преодолеть свои денежные трудности, предоставив прозрачные и выгодные условия кредитования. Доверьтесь нашей компании, и мы поможем вам в решении ваших финансовых проблем.
На сайте https://luxbabycar.ru представлен каталог детских авто. Ассортимент постоянно обновляется. Здесь вы найдете: джипы, квадроциклы, багги, спецтехнику, толокар и многое другое высокого качества и по конкурентоспособным ценам. У нас самый широкий выбор детского автопарка. На сайте вы можете ознакомиться с фото-отзывами, а также посмотреть видео-обзор. Проконсультируйтесь со специалистом прямо сейчас. Весь товар прошел проверку качества, вы можете быть уверены за свой новый автомобиль.
В сегодняшнем обществе финансовые сложности не редкость, и каждый человек может оказаться в ситуации, когда потребуется дополнительная финансовая помощь. На сайте [url=https://moneybel.ru/]деньги в долг[/url] помогут преодолеть эти трудности, принося простой и удобный способ получения кредита. Мы осознаём, что время играет ключевую роль, поэтому наш процесс обработки заявок максимально быстр и эффективен. В качестве представителя Манибел, наша цель – помочь каждому клиенту преодолеть финансовые затруднения, предложив ясные и выгодные условия займа. Доверьтесь нашей компании, и мы поможем вам в решении ваших финансовых проблем.
В сегодняшнем обществе финансовые сложности не редкость, и каждый человек может оказаться в ситуации, когда потребуется дополнительная финансовая помощь. На сайте [url=https://moneybel.ru/]деньги в кредит[/url] помогут преодолеть эти трудности, предоставляя простой и удобный способ получения кредита. Мы осознаём, что времени на решение проблемы не хватает, поэтому наш процесс обработки заявок максимально быстр и эффективен. В роли представителя Манибел, наша цель – помочь каждому клиенту преодолеть финансовые затруднения, предложив ясные и выгодные условия займа. Доверьтесь нашей компании, и мы поможем вам в решении ваших финансовых проблем.
В сегодняшнем обществе финансовые трудности не редкость, и каждый из нас может оказаться в ситуации, когда необходима дополнительная финансовая поддержка. На сайте [url=https://moneybel.ru/]взять деньги в долг[/url] помогут решить эти проблемы, принося простой и лёгкий метод займа. Мы осознаём, что время играет ключевую роль, поэтому наш процесс обработки заявок максимально быстр и эффективен. В качестве представителя Манибел, мы нацелены на то, чтобы помочь каждому клиенту преодолеть свои денежные трудности, предложив ясные и выгодные условия займа. Доверьтесь нам, и мы поможем вам в решении ваших финансовых проблем.
В настоящей эпохе финансовые трудности не редкость, и каждый человек может оказаться в ситуации, когда потребуется дополнительная финансовая помощь. На сайте [url=https://moneybel.ru/]деньги в долг на карту[/url] помогут решить эти проблемы, предоставляя простой и удобный способ получения кредита. Мы понимаем, что время играет ключевую роль, поэтому мы обрабатываем заявки оперативно и эффективно. В роли представителя Манибел, мы нацелены на то, чтобы помочь каждому клиенту преодолеть свои денежные трудности, предложив ясные и выгодные условия займа. Доверьтесь нам, и мы поможем вам в решении ваших финансовых проблем.
[url=http://bmtadalafil.online/]generic tadalafil 2017[/url]
В настоящей экономической обстановке, где неожиданные финансовые обязательства могут возникнуть в любое время, поиск надежного и удобного источника финансовой помощи становится все более критическим. Предложение [url=https://zaim-minsk.ru/]деньги в долг на карту[/url] предлагает возможность рассчитывать на быстрое и беззаботное получение необходимых средств без лишних трудностей. Мы ценим ваше время и обеспечиваем оперативную обработку всех запросов. В качестве вашего финансового партнера, наша главная задача состоит в том, чтобы помочь вам преодолеть текущие финансовые препятствия, предоставляя ясные и выгодные условия кредитования. Доверьтесь нам в вашем финансовом путешествии, и мы обеспечим вас надежной поддержкой на каждом этапе.
В настоящей экономической обстановке, где неожиданные финансовые обязательства могут возникнуть в любое время, поиск надежного и удобного источника финансовой помощи становится все более критическим. Предложение [url=https://zaim-minsk.ru/]деньги в долг онлайн[/url] предлагает возможность рассчитывать на быстрое и беззаботное получение необходимых средств без лишних трудностей. Мы ценим ваше время и обеспечиваем оперативную обработку всех запросов. В качестве вашего финансового партнера, наша главная задача состоит в том, чтобы помочь вам преодолеть текущие финансовые препятствия, предоставляя ясные и выгодные условия кредитования. Доверьтесь нам в вашем финансовом путешествии, и мы обеспечим вас надежной поддержкой на каждом этапе.
В настоящей экономической обстановке, где неожиданные финансовые обязательства могут возникнуть в любое время, поиск надежного и удобного источника финансовой помощи становится все более критическим. Предложение [url=https://zaim-minsk.ru/]займ в РБ[/url] предлагает возможность рассчитывать на быстрое и беззаботное получение необходимых средств без лишних трудностей. Мы ценим ваше время и обеспечиваем оперативную обработку всех запросов. В качестве вашего финансового партнера, наша главная задача состоит в том, чтобы помочь вам преодолеть текущие финансовые препятствия, предоставляя ясные и выгодные условия кредитования. Доверьтесь нам в вашем финансовом путешествии, и мы обеспечим вас надежной поддержкой на каждом этапе.
В настоящей экономической обстановке, где неожиданные финансовые обязательства могут возникнуть в любое время, поиск надежного и удобного источника финансовой помощи становится все более критическим. Предложение [url=https://zaim-minsk.ru/]взять деньги в долг[/url] предлагает возможность рассчитывать на быстрое и беззаботное получение необходимых средств без лишних трудностей. Мы ценим ваше время и обеспечиваем оперативную обработку всех запросов. В качестве вашего финансового партнера, наша главная задача состоит в том, чтобы помочь вам преодолеть текущие финансовые препятствия, предоставляя ясные и выгодные условия кредитования. Доверьтесь нам в вашем финансовом путешествии, и мы обеспечим вас надежной поддержкой на каждом этапе.
В настоящей экономической обстановке, где неожиданные финансовые обязательства могут возникнуть в любое время, поиск надежного и удобного источника финансовой помощи становится все более критическим. Предложение [url=https://zaim-minsk.ru/]взять деньги[/url] предлагает возможность рассчитывать на быстрое и беззаботное получение необходимых средств без лишних трудностей. Мы ценим ваше время и обеспечиваем оперативную обработку всех запросов. В качестве вашего финансового партнера, наша главная задача состоит в том, чтобы помочь вам преодолеть текущие финансовые препятствия, предоставляя ясные и выгодные условия кредитования. Доверьтесь нам в вашем финансовом путешествии, и мы обеспечим вас надежной поддержкой на каждом этапе.
На сайте https://vostrilov.com/ запишитесь на консультацию для того, чтобы воспользоваться услугами популярного, надежного пластического хирурга Вострилова Ивана Михайловича. Прямо сейчас ознакомьтесь с результатами, на которые вы сможете рассчитывать после проведения манипуляций. Врач работает с 2015 года. Если остались вопросы, то задайте их менеджеру. Для этого введите свои данные в форму, а затем вам перезвонит менеджер. Теперь и вы сможете заполучить внешность своей мечты и радоваться отражению в зеркале.
В настоящей экономической обстановке, где неожиданные финансовые обязательства могут возникнуть в любое время, поиск надежного и удобного источника финансовой помощи становится все более критическим. Предложение [url=https://zaim-minsk.ru/]деньги в долг[/url] предлагает возможность рассчитывать на быстрое и беззаботное получение необходимых средств без лишних трудностей. Мы ценим ваше время и обеспечиваем оперативную обработку всех запросов. В качестве вашего финансового партнера, наша главная задача состоит в том, чтобы помочь вам преодолеть текущие финансовые препятствия, предоставляя ясные и выгодные условия кредитования. Доверьтесь нам в вашем финансовом путешествии, и мы обеспечим вас надежной поддержкой на каждом этапе.
В настоящей экономической обстановке, где неожиданные финансовые обязательства могут возникнуть в любое время, поиск надежного и удобного источника финансовой помощи становится все более критическим. Предложение [url=https://zaim-minsk.ru/]взять денег в долг срочно[/url] предлагает возможность рассчитывать на быстрое и беззаботное получение необходимых средств без лишних трудностей. Мы ценим ваше время и обеспечиваем оперативную обработку всех запросов. В качестве вашего финансового партнера, наша главная задача состоит в том, чтобы помочь вам преодолеть текущие финансовые препятствия, предоставляя ясные и выгодные условия кредитования. Доверьтесь нам в вашем финансовом путешествии, и мы обеспечим вас надежной поддержкой на каждом этапе.
nationalities, religion and traditions. Therefore, when you order girls from Hackney escorts, you can pick an Asian, Slavic, with the high quality escort services including sex, erotic massage and striptease. Girls who have dedicated themselves to the escort in south east london
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]купить ленты конвейерные на Bellenty[/url] помогут консультанты магазина. Благодаря нашему практике и профессионализму мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам стандартам качества и эффективности. Мы производим индивидуальные решения, учитывая особые потребности наших клиентов. Независимо от размера, материала или технических характеристик, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Хотите смотреть фильмы и сериалы онлайн в лучшем качестве? Kinogo вам в этом поможет. Здесь представлено много фильмов, которые можно абсолютно бесплатно посмотреть. https://kinogo-la.net – место, где есть возможность найти интересное кино. Сайт безопасный и имеет понятный интерфейс. Выглядит он превосходно, и пользоваться им достаточно просто. На портале есть множество информации о фильмах, ознакомиться с ней можно в любое удобное время. Содержимое этого сервиса всегда актуально. Устраивайтесь поудобнее, и смотрите любимое кино!
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]стоимость транспортерных лент на Bellenty[/url] помогут консультанты интернет-магазина. Благодаря нашему практике и профессионализму мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам стандартам качества и эффективности. Мы изготавливаем индивидуальные решения, учитывая особые потребности наших заказчиков. Независимо от размера, материала или технических характеристик, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]купить конвейерную ленту на Bellenty[/url] помогут консультанты интернет-магазина. Благодаря нашему опыту и профессионализму мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам стандартам качества и эффективности. Мы разрабатываем индивидуальные решения, учитывая особые потребности наших клиентов. Независимо от размера, материала или технических характеристик, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]заказать ленты транспортерные в Минске[/url] помогут консультанты интернет-магазина. Благодаря нашему опыту и профессионализму мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам стандартам качества и эффективности. Мы производим индивидуальные решения, учитывая особые потребности наших заказчиков. Независимо от размера, состава или технических характеристик, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]оформить ленту транспортерную на Bellenty[/url] помогут консультанты магазина. Благодаря нашему практике и профессионализму мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам качества и эффективности. Мы изготавливаем индивидуальные решения, учитывая особые потребности наших клиентов. Независимо от величины, материала или спецификаций, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]конвейерная лента на Bellenty[/url] помогут консультанты интернет-магазина. Благодаря нашему практике и профессионализму мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам стандартам качества и эффективности. Мы изготавливаем индивидуальные решения, учитывая уникальные потребности наших клиентов. Независимо от величины, материала или технических характеристик, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]bellenty.by[/url] помогут консультанты интернет-магазина. Благодаря нашему опыту и компетентности мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам стандартам качества и эффективности. Мы изготавливаем индивидуальные решения, учитывая особые потребности наших клиентов. Независимо от размера, материала или спецификаций, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]ленты конвейерные[/url] помогут консультанты магазина. Благодаря нашему практике и профессионализму мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам качества и эффективности. Мы разрабатываем индивидуальные решения, учитывая уникальные потребности наших клиентов. Независимо от размера, материала или технических характеристик, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
В настоящее время наши дни могут содержать неожиданные расходы и финансовые вызовы, и в такие моменты каждый ищет помощь. На сайте [url=https://brokers-group.ru/]взять денег в долг срочно[/url] вам помогут без избыточной бюрократии. В своей роли представителя я стремлюсь распространить эту информацию, чтобы помочь тем, кто столкнулся с временными трудностями. Наша система обеспечивает честные условия и быструю обработку заявок, чтобы каждый мог решить свои денежные проблемы быстро и без лишних хлопот.
В настоящее время наши дни могут содержать внезапные издержки и финансовые вызовы, и в такие моменты каждый ищет надежную поддержку. На сайте [url=https://brokers-group.ru/]взять деньги[/url] вам помогут без лишних сложностей. В своей роли агента я стремлюсь распространить эту информацию, чтобы помочь тем, кто столкнулся с временными трудностями. Наша система обеспечивает честные условия и эффективное рассмотрение запросов, чтобы каждый мог разрешить свои финансовые вопросы оперативно и без лишних затрат времени.
В настоящее время наши дни могут содержать неожиданные расходы и экономические трудности, и в такие моменты каждый ищет помощь. На сайте [url=https://brokers-group.ru/]деньги в долг[/url] вам помогут без избыточной бюрократии. В своей роли представителя я стремлюсь поделиться этими сведениями, чтобы помочь людям в сложной ситуации. Наша платформа обеспечивает прозрачные условия и эффективное рассмотрение запросов, чтобы каждый мог разрешить свои финансовые вопросы быстро и без лишних хлопот.
В настоящее время наши дни могут содержать неожиданные расходы и экономические трудности, и в такие моменты каждый ищет помощь. На сайте [url=https://brokers-group.ru/]получить займ в Минске[/url] вам помогут без лишних сложностей. В своей роли представителя я стремлюсь распространить эту информацию, чтобы помочь людям в сложной ситуации. Наша система обеспечивает прозрачные условия и эффективное рассмотрение запросов, чтобы каждый мог разрешить свои финансовые вопросы быстро и без лишних хлопот.
В настоящее время наши дни могут содержать внезапные издержки и финансовые вызовы, и в такие моменты каждый ищет помощь. На сайте [url=https://brokers-group.ru/]взять деньги в долг[/url] вам помогут без лишних сложностей. В своей роли агента я стремлюсь распространить эту информацию, чтобы помочь людям в сложной ситуации. Наша система обеспечивает прозрачные условия и быструю обработку заявок, чтобы каждый мог разрешить свои финансовые вопросы быстро и без лишних хлопот.
В настоящее время наши дни могут содержать внезапные издержки и финансовые вызовы, и в такие моменты каждый ищет помощь. На сайте [url=https://brokers-group.ru/]оформить займ[/url] вам помогут без избыточной бюрократии. В своей роли представителя я стремлюсь распространить эту информацию, чтобы помочь тем, кто столкнулся с временными трудностями. Наша система обеспечивает честные условия и быструю обработку заявок, чтобы каждый мог решить свои денежные проблемы быстро и без лишних хлопот.
В настоящее время наши дни могут содержать неожиданные расходы и финансовые вызовы, и в такие моменты каждый ищет надежную поддержку. На сайте [url=https://brokers-group.ru/]деньги в долг[/url] вам помогут без избыточной бюрократии. В своей роли представителя я стремлюсь распространить эту информацию, чтобы помочь тем, кто столкнулся с временными трудностями. Наша платформа обеспечивает честные условия и эффективное рассмотрение запросов, чтобы каждый мог разрешить свои финансовые вопросы быстро и без лишних хлопот.
В настоящее время наши дни могут содержать неожиданные расходы и экономические трудности, и в такие моменты каждый ищет надежную поддержку. На сайте [url=https://brokers-group.ru/]Брокерс Групп[/url] вам помогут без лишних сложностей. В своей роли агента я стремлюсь поделиться этими сведениями, чтобы помочь тем, кто столкнулся с временными трудностями. Наша платформа обеспечивает честные условия и быструю обработку заявок, чтобы каждый мог разрешить свои финансовые вопросы оперативно и без лишних затрат времени.
В наше время многие из нас сталкиваются с непредвиденными расходами и денежными трудностями, и в такие моменты важно иметь доступ к дополнительным средствам. На сайте [url=https://dengizaimy.ru/]получить займ в Минске[/url] вам помогут быстро и удобно. В качестве представителя Брокерс Групп, я рад делиться этой важной информацией, чтобы помочь каждому, кто нуждается в финансовой поддержке. Наша платформа гарантирует прозрачные условия и оперативное рассмотрение запросов, чтобы каждый мог решить свои финансовые вопросы с минимальными временными затратами.
В наше время многие из нас сталкиваются с внезапными затратами и денежными трудностями, и в такие моменты важно иметь возможность взять кредит. На сайте [url=https://dengizaimy.ru/]займ на карту[/url] вам помогут с минимальными усилиями. В качестве представителя Брокерс Групп, я рад делиться этой информацией, чтобы помочь людям, кто столкнулся с финансовыми трудностями. Наша платформа гарантирует честные условия и оперативное рассмотрение запросов, чтобы каждый мог решить свои финансовые вопросы с минимальными временными затратами.
В наше время многие из нас сталкиваются с внезапными затратами и денежными трудностями, и в такие моменты важно иметь возможность взять кредит. На сайте [url=https://dengizaimy.ru/]оформить займ в Минске[/url] вам помогут с минимальными усилиями. В качестве официального представителя Брокерс Групп, я с удовольствием делиться этой информацией, чтобы помочь людям, кто столкнулся с финансовыми трудностями. Наша система гарантирует честные условия и быструю обработку заявок, чтобы каждый мог решить свои финансовые вопросы с минимальными временными затратами.
На сайте https://taker10.casino сыграйте в интересные, увлекательные и удивительные игры, которые произведут на вас впечатление своей динамикой, ярким оформлением. Есть Live-игры, которые позволят погрузиться в атмосферу азартных игр, топовые слоты, которые помогут попытать удачу и получить огромный выигрыш. Обязательно посетите раздел с топовыми играми, которые выбирает большинство. Воспользуйтесь возможностью заработать до 15% от депозитов рефералов. Вы сможете получать до 100 рублей ежедневно в качестве бонуса.
В наше время многие из нас сталкиваются с внезапными затратами и экономическими вызовами, и в такие моменты важно иметь доступ к дополнительным средствам. На сайте [url=https://dengizaimy.ru/]займ денег минск[/url] вам помогут с минимальными усилиями. В качестве представителя Брокерс Групп, я с удовольствием делиться этой важной информацией, чтобы помочь людям, кто столкнулся с финансовыми трудностями. Наша платформа гарантирует прозрачные условия и оперативное рассмотрение запросов, чтобы каждый мог разрешить свои денежные проблемы с минимальными временными затратами.
В наше время многие из нас сталкиваются с внезапными затратами и денежными трудностями, и в такие моменты важно иметь возможность взять кредит. На сайте [url=https://dengizaimy.ru/]деньги в долг онлайн[/url] вам помогут с минимальными усилиями. В качестве представителя Брокерс Групп, я с удовольствием делиться этой информацией, чтобы помочь людям, кто столкнулся с финансовыми трудностями. Наша система гарантирует честные условия и оперативное рассмотрение запросов, чтобы каждый мог решить свои финансовые вопросы быстро и эффективно.
В наше время многие из нас сталкиваются с внезапными затратами и денежными трудностями, и в такие моменты важно иметь доступ к дополнительным средствам. На сайте [url=https://dengizaimy.ru/]займ на карту[/url] вам помогут с минимальными усилиями. В качестве официального представителя Брокерс Групп, я рад делиться этой важной информацией, чтобы помочь каждому, кто столкнулся с финансовыми трудностями. Наша система гарантирует прозрачные условия и оперативное рассмотрение запросов, чтобы каждый мог решить свои финансовые вопросы с минимальными временными затратами.
Зайдите на сайт https://luckyfood.ru/ где вы сможете купить мясо для собак с быстрой доставкой или самовывозом в Москве. Действует система скидок. Ознакомьтесь с большим каталогом продукции – говядина, баранина, индейка, субпродукты и многое другое. Порадуйте своего питомца, а легкость оформления заказа обязательно понравится!
сео оптимизатор https://seo-goody.ru/
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]транспортерные ленты на Bellenty[/url] помогут консультанты магазина. Благодаря нашему опыту и компетентности мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам стандартам качества и эффективности. Мы производим индивидуальные решения, учитывая уникальные потребности наших заказчиков. Независимо от размера, материала или технических характеристик, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]оформить ленты транспортерные на Bellenty.by[/url] помогут консультанты магазина. Благодаря нашему опыту и компетентности мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам качества и эффективности. Мы производим индивидуальные решения, учитывая особые потребности наших клиентов. Независимо от величины, состава или технических характеристик, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]цена ленты транспортерной в Минске[/url] помогут консультанты магазина. Благодаря нашему практике и профессионализму мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам стандартам качества и эффективности. Мы изготавливаем индивидуальные решения, учитывая особые потребности наших заказчиков. Независимо от размера, состава или спецификаций, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]оформить транспортерную ленту на Bellenty[/url] помогут консультанты магазина. Благодаря нашему опыту и профессионализму мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам качества и эффективности. Мы разрабатываем индивидуальные решения, учитывая уникальные потребности наших заказчиков. Независимо от величины, состава или спецификаций, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]заказать конвейерную ленту на Bellenty.by[/url] помогут консультанты интернет-магазина. Благодаря нашему практике и профессионализму мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам стандартам качества и эффективности. Мы изготавливаем индивидуальные решения, учитывая уникальные потребности наших заказчиков. Независимо от размера, материала или спецификаций, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]заказать ленту конвейерную в Минске[/url] помогут консультанты магазина. Благодаря нашему опыту и компетентности мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам стандартам качества и эффективности. Мы изготавливаем индивидуальные решения, учитывая уникальные потребности наших заказчиков. Независимо от размера, материала или спецификаций, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]оформить конвейерную ленту на Bellenty[/url] помогут консультанты магазина. Благодаря нашему практике и профессионализму мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам качества и эффективности. Мы производим индивидуальные решения, учитывая особые потребности наших клиентов. Независимо от размера, состава или спецификаций, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Закажите SEO продвижение сайта https://seo116.ru/ в Яндекс и Google под ключ в Москве и по всей России от экспертов. Увеличение трафика, рост клиентов, онлайн поддержка. Комплексное продвижение сайтов с гарантией!
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]стоимость конвейерной ленты[/url] помогут консультанты магазина. Благодаря нашему опыту и компетентности мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам качества и эффективности. Мы разрабатываем индивидуальные решения, учитывая уникальные потребности наших клиентов. Независимо от размера, состава или технических характеристик, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]цена конвейерной ленты на Bellenty.by[/url] помогут консультанты интернет-магазина. Благодаря нашему практике и профессионализму мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам качества и эффективности. Мы производим индивидуальные решения, учитывая уникальные потребности наших клиентов. Независимо от размера, состава или спецификаций, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
На сайте http://waltzprof.com вы можете на выгодных условиях приобрести стальной профиль в широком ассортименте. Они предназначены для лофт перегородок, фасадных конструкций, удобной и быстрой сборки ограждающих преград и другое. Компания использует передовые технологии для выпуска качественной продукции. Покупка от производителя – это прекрасный способ сэкономить на услугах посредника. Подробнее ознакомиться с продукцией и посмотреть информацию о компании, вы можете на сайте. Закажите обратный звонок, и менеджеры вас проконсультируют по всем вопросам.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]оформить транспортерные ленты[/url] помогут консультанты магазина. Благодаря нашему опыту и компетентности мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам качества и эффективности. Мы производим индивидуальные решения, учитывая особые потребности наших клиентов. Независимо от величины, материала или технических характеристик, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]конвейерные ленты на Bellenty.by[/url] помогут консультанты интернет-магазина. Благодаря нашему опыту и профессионализму мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам качества и эффективности. Мы изготавливаем индивидуальные решения, учитывая уникальные потребности наших клиентов. Независимо от размера, состава или спецификаций, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]http://bellenty.by/[/url] помогут консультанты магазина. Благодаря нашему практике и профессионализму мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам качества и эффективности. Мы производим индивидуальные решения, учитывая особые потребности наших клиентов. Независимо от размера, состава или спецификаций, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]цена ленты транспортерной на Bellenty.by[/url] помогут консультанты интернет-магазина. Благодаря нашему практике и компетентности мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам качества и эффективности. Мы производим индивидуальные решения, учитывая особые потребности наших клиентов. Независимо от величины, материала или спецификаций, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Bellenty – ваш надёжный союзник в области конвейерных решений. Для вас [url=http://bellenty.by/]купить ленту конвейерную на Bellenty[/url] помогут консультанты магазина. Благодаря нашему опыту и профессионализму мы гарантируем, что каждая лента, предлагаемая нами, соответствует высочайшим стандартам стандартам качества и эффективности. Мы производим индивидуальные решения, учитывая особые потребности наших заказчиков. Независимо от величины, состава или технических характеристик, Bellenty обеспечивает идеальное решение для вашего конвейерного оборудования, даря вашему бизнесу надёжность и эффективность.
Посетите сайт https://fulldog.ru/ где вы найдете широкий ассортимент мяса для собак. Можно воспользоваться доставкой по Москве и области или забрать самовывозом. Мясо для собак – основа полезного рациона для вашего питомца, а спецпредложения сделают покупку еще более выгодной.
В настоящее время наши дни могут содержать неожиданные расходы и финансовые вызовы, и в такие моменты каждый ищет надежную поддержку. На сайте [url=https://brokers-group.ru/]brokers-group.ru[/url] вам помогут без избыточной бюрократии. В своей роли агента я стремлюсь распространить эту информацию, чтобы помочь тем, кто столкнулся с временными трудностями. Наша система обеспечивает прозрачные условия и быструю обработку заявок, чтобы каждый мог решить свои денежные проблемы оперативно и без лишних затрат времени.
[url=http://lisinoprill.com/]lisinopril tab 20mg cost[/url]
В настоящее время наши дни могут содержать внезапные издержки и экономические трудности, и в такие моменты каждый ищет помощь. На сайте [url=https://brokers-group.ru/]займ на карту[/url] вам помогут без избыточной бюрократии. В своей роли представителя я стремлюсь поделиться этими сведениями, чтобы помочь людям в сложной ситуации. Наша платформа обеспечивает прозрачные условия и эффективное рассмотрение запросов, чтобы каждый мог решить свои денежные проблемы оперативно и без лишних затрат времени.
На сайте https://vk.com/wall-101758973_774 вы сможете оперативно заказать печать в Белгороде. К заказу доступны различные виды форматов печати. Выкройки, чертежи будут созданы непосредственно при вас. Обязательно закажите плоттерную резку. Есть возможность воспользоваться услугой по индивидуальному дизайну. Все изготавливается в минимальные сроки. Доступна печать на футболке номеров, текста, фотографий. Компания работает по удобному для всех графику и на инновационном, высокотехнологичном оборудовании. Специалисты выполнят все работы согласно требованиям.
В настоящее время наши дни могут содержать неожиданные расходы и экономические трудности, и в такие моменты каждый ищет надежную поддержку. На сайте [url=https://brokers-group.ru/]brokers-group.ru[/url] вам помогут без избыточной бюрократии. В своей роли агента я стремлюсь распространить эту информацию, чтобы помочь тем, кто столкнулся с временными трудностями. Наша система обеспечивает честные условия и эффективное рассмотрение запросов, чтобы каждый мог решить свои денежные проблемы оперативно и без лишних затрат времени.
В настоящее время наши дни могут содержать внезапные издержки и финансовые вызовы, и в такие моменты каждый ищет помощь. На сайте [url=https://brokers-group.ru/]взять деньги в долг[/url] вам помогут без избыточной бюрократии. В своей роли представителя я стремлюсь поделиться этими сведениями, чтобы помочь людям в сложной ситуации. Наша система обеспечивает прозрачные условия и быструю обработку заявок, чтобы каждый мог решить свои денежные проблемы оперативно и без лишних затрат времени.
В настоящее время наши дни могут содержать неожиданные расходы и экономические трудности, и в такие моменты каждый ищет помощь. На сайте [url=https://brokers-group.ru/]займ в Минске[/url] вам помогут без лишних сложностей. В своей роли представителя я стремлюсь распространить эту информацию, чтобы помочь людям в сложной ситуации. Наша система обеспечивает честные условия и быструю обработку заявок, чтобы каждый мог решить свои денежные проблемы быстро и без лишних хлопот.
В настоящее время наши дни могут содержать внезапные издержки и экономические трудности, и в такие моменты каждый ищет надежную поддержку. На сайте [url=https://brokers-group.ru/]получить деньги[/url] вам помогут без избыточной бюрократии. В своей роли агента я стремлюсь поделиться этими сведениями, чтобы помочь тем, кто столкнулся с временными трудностями. Наша система обеспечивает честные условия и быструю обработку заявок, чтобы каждый мог разрешить свои финансовые вопросы быстро и без лишних хлопот.
В настоящее время наши дни могут содержать неожиданные расходы и финансовые вызовы, и в такие моменты каждый ищет надежную поддержку. На сайте [url=https://brokers-group.ru/]Брокерс Групп[/url] вам помогут без избыточной бюрократии. В своей роли представителя я стремлюсь поделиться этими сведениями, чтобы помочь тем, кто столкнулся с временными трудностями. Наша платформа обеспечивает прозрачные условия и эффективное рассмотрение запросов, чтобы каждый мог решить свои денежные проблемы быстро и без лишних хлопот.
Привет всем!
От политики до спорта, от экономики до науки – мы предлагаем вам самую актуальную информацию из разных областей жизни. Наши новости помогут вам понять текущую ситуацию и принимать информированные решения.
Наши новости помогут вам быть в курсе самых важных событий в Украине и мире. Мы предлагаем вам объективную информацию, которая поможет вам понять происходящее вокруг.
Все самое лучшее на сайте https://kompromis.info/auto/
[url=https://kompromis.info/economika/]новости Экономики[/url]
авто новости
новости Украины за день
дтп видео
Удачи!
купить больничный лист в москве
В наше время многие из нас сталкиваются с внезапными затратами и экономическими вызовами, и в такие моменты важно иметь возможность взять кредит. На сайте [url=https://dengizaimy.ru/]dengizaimy.ru[/url] вам помогут быстро и удобно. В качестве официального представителя Брокерс Групп, я с удовольствием делиться этой информацией, чтобы помочь каждому, кто столкнулся с финансовыми трудностями. Наша система гарантирует честные условия и оперативное рассмотрение запросов, чтобы каждый мог разрешить свои денежные проблемы быстро и эффективно.
В наше время многие из нас сталкиваются с непредвиденными расходами и экономическими вызовами, и в такие моменты важно иметь доступ к дополнительным средствам. На сайте [url=https://dengizaimy.ru/]деньги в долг[/url] вам помогут с минимальными усилиями. В качестве представителя Брокерс Групп, я с удовольствием делиться этой важной информацией, чтобы помочь людям, кто нуждается в финансовой поддержке. Наша платформа гарантирует прозрачные условия и оперативное рассмотрение запросов, чтобы каждый мог решить свои финансовые вопросы быстро и эффективно.
В наше время многие из нас сталкиваются с внезапными затратами и экономическими вызовами, и в такие моменты важно иметь возможность взять кредит. На сайте [url=https://dengizaimy.ru/]взять деньги в долг[/url] вам помогут с минимальными усилиями. В качестве представителя Брокерс Групп, я с удовольствием делиться этой важной информацией, чтобы помочь людям, кто нуждается в финансовой поддержке. Наша платформа гарантирует прозрачные условия и быструю обработку заявок, чтобы каждый мог решить свои финансовые вопросы с минимальными временными затратами.
В наше время многие из нас сталкиваются с внезапными затратами и денежными трудностями, и в такие моменты важно иметь доступ к дополнительным средствам. На сайте [url=https://dengizaimy.ru/]взять деньги в долг[/url] вам помогут быстро и удобно. В качестве официального представителя Брокерс Групп, я рад делиться этой информацией, чтобы помочь людям, кто нуждается в финансовой поддержке. Наша платформа гарантирует прозрачные условия и оперативное рассмотрение запросов, чтобы каждый мог решить свои финансовые вопросы быстро и эффективно.
В наше время многие из нас сталкиваются с непредвиденными расходами и экономическими вызовами, и в такие моменты важно иметь доступ к дополнительным средствам. На сайте [url=https://dengizaimy.ru/]получить деньги в долг[/url] вам помогут с минимальными усилиями. В качестве представителя Брокерс Групп, я рад делиться этой информацией, чтобы помочь каждому, кто столкнулся с финансовыми трудностями. Наша платформа гарантирует прозрачные условия и быструю обработку заявок, чтобы каждый мог решить свои финансовые вопросы быстро и эффективно.
В наше время многие из нас сталкиваются с внезапными затратами и денежными трудностями, и в такие моменты важно иметь возможность взять кредит. На сайте [url=https://dengizaimy.ru/]займ денег[/url] вам помогут быстро и удобно. В качестве представителя Брокерс Групп, я с удовольствием делиться этой информацией, чтобы помочь людям, кто столкнулся с финансовыми трудностями. Наша платформа гарантирует прозрачные условия и оперативное рассмотрение запросов, чтобы каждый мог разрешить свои денежные проблемы быстро и эффективно.
В наше время многие из нас сталкиваются с непредвиденными расходами и экономическими вызовами, и в такие моменты важно иметь доступ к дополнительным средствам. На сайте [url=https://dengizaimy.ru/]займ денег[/url] вам помогут быстро и удобно. В качестве официального представителя Брокерс Групп, я рад делиться этой информацией, чтобы помочь каждому, кто нуждается в финансовой поддержке. Наша платформа гарантирует честные условия и оперативное рассмотрение запросов, чтобы каждый мог разрешить свои денежные проблемы быстро и эффективно.
В наше время многие из нас сталкиваются с непредвиденными расходами и денежными трудностями, и в такие моменты важно иметь возможность взять кредит. На сайте [url=https://dengizaimy.ru/]получить деньги в долг в Минске[/url] вам помогут быстро и удобно. В качестве представителя Брокерс Групп, я с удовольствием делиться этой важной информацией, чтобы помочь людям, кто нуждается в финансовой поддержке. Наша система гарантирует прозрачные условия и быструю обработку заявок, чтобы каждый мог решить свои финансовые вопросы с минимальными временными затратами.
В настоящей эпохе финансовые трудности встречаются часто, и каждый из нас может оказаться в ситуации, когда необходима дополнительная финансовая поддержка. На сайте [url=https://moneybel.ru/]МаниБел[/url] помогут решить эти проблемы, предоставляя простой и удобный способ получения кредита. Мы понимаем, что времени на решение проблемы не хватает, поэтому мы обрабатываем заявки оперативно и эффективно. В качестве представителя Манибел, наша цель – помочь каждому клиенту преодолеть финансовые затруднения, предоставив прозрачные и выгодные условия кредитования. Доверьтесь нашей компании, и мы решим ваши финансовые проблемы.
В сегодняшнем обществе финансовые сложности не редкость, и каждый из нас может оказаться в ситуации, когда потребуется дополнительная финансовая помощь. На сайте [url=https://moneybel.ru/]деньги в долг[/url] помогут преодолеть эти трудности, принося простой и удобный способ займа. Мы понимаем, что времени на решение проблемы не хватает, поэтому наш процесс обработки заявок максимально быстр и эффективен. В качестве представителя Манибел, мы нацелены на то, чтобы помочь каждому клиенту преодолеть свои денежные трудности, предложив ясные и выгодные условия займа. Доверьтесь нам, и мы решим ваши финансовые проблемы.
утилизация мусора Промышленный район
В сегодняшнем обществе финансовые трудности встречаются часто, и каждый из нас может оказаться в ситуации, когда потребуется дополнительная финансовая помощь. На сайте [url=https://moneybel.ru/]взять деньги в долг[/url] помогут решить эти проблемы, предоставляя простой и лёгкий метод займа. Мы понимаем, что времени на решение проблемы не хватает, поэтому мы обрабатываем заявки оперативно и эффективно. В качестве представителя Манибел, мы нацелены на то, чтобы помочь каждому клиенту преодолеть свои денежные трудности, предоставив прозрачные и выгодные условия кредитования. Доверьтесь нам, и мы решим ваши финансовые проблемы.
В сегодняшнем обществе финансовые трудности не редкость, и каждый из нас может оказаться в ситуации, когда необходима дополнительная финансовая поддержка. На сайте [url=https://moneybel.ru/]moneybel[/url] помогут решить эти проблемы, принося простой и лёгкий метод получения кредита. Мы осознаём, что время играет ключевую роль, поэтому наш процесс обработки заявок максимально быстр и эффективен. В роли представителя Манибел, наша цель – помочь каждому клиенту преодолеть финансовые затруднения, предложив ясные и выгодные условия займа. Доверьтесь нам, и мы поможем вам в решении ваших финансовых проблем.
Запускайте свежую версию приложения БК Олимп для Android и измените свои стандарты в мире ставок! [url=https://apk-olimp.ru/]Скачать Olimp bet для андроид[/url] доступно для всех. С передовым дизайном и ускоренной загрузкой данных, доступ к многочисленным спортивным событиям становится игрой. Управление аккаунтом и перемещение по разделам теперь настолько просты, что каждая ставка превращается в радость. Установите последнюю версию прямо сейчас и начните выигрывать с БК Олимп, где каждый шаг может привести к значимому триумфу!
Запускайте свежую версию приложения БК Олимп для Android и переопределите свои стандарты в мире ставок! [url=https://apk-olimp.ru/]Приложение Olimp bet[/url] уже сейчас. С передовым дизайном и быстрой загрузкой данных, доступ к многочисленным спортивным событиям становится игрой. Ведение счета и перемещение по разделам теперь настолько просты, что каждая ставка превращается в удовольствие. Установите последнюю версию прямо сейчас и начните добиваться успеха с БК Олимп, где каждый шаг может привести к большой победе!
Всё о радиаторах отопления https://heat-komfort.ru/ – выбор радиатора, монтаж, обслуживание.
Запускайте новую версию приложения БК Олимп для Android и измените свои стандарты в мире ставок! [url=https://apk-olimp.ru/]Скачать Олимп на андроид[/url] уже сейчас. С передовым дизайном и ускоренной загрузкой данных, доступ к различным спортивным событиям становится игрой. Управление аккаунтом и навигация по приложению теперь настолько просты, что каждая ставка превращается в радость. Скачайте последнюю версию прямо сейчас и начните выигрывать с БК Олимп, где каждый шаг может привести к значимому триумфу!
Запускайте новую версию приложения БК Олимп для Android и переопределите свои стандарты в мире ставок! [url=https://apk-olimp.ru/]Скачать Олимп бесплатно[/url] уже сегодня. С современным дизайном и быстрой загрузкой данных, доступ к различным спортивным событиям становится игрой. Управление аккаунтом и перемещение по разделам теперь настолько просты, что каждая ставка превращается в удовольствие. Скачайте последнюю версию без задержек и начните выигрывать с БК Олимп, где каждый шаг может привести к значимому триумфу!
Welcome to OTunnels https://otunnels.com/ – your destination for unique ear tunnels and plugs! Explore our wide range of stylish and high-quality ear adornments. We offer a diverse selection of designs, including gothic motifs, cyberpunk aesthetics, geometric patterns, and much more. Our products are crafted using innovative materials, ensuring safety and comfort during wear. Dive into our collection and discover the perfect statement piece to elevate your style with OTunnels!
Запускайте новую версию приложения БК Олимп для Android и переопределите свои стандарты в мире ставок! [url=https://apk-olimp.ru/]Скачать приложение букмекерской Олимп[/url] сегодня. С передовым дизайном и быстрой загрузкой данных, доступ к различным спортивным событиям становится игрой. Ведение счета и навигация по приложению теперь так удобны, что каждая ставка превращается в удовольствие. Установите последнюю версию без задержек и начните выигрывать с БК Олимп, где каждый шаг может привести к большой победе!
Запускайте свежую версию приложения БК Олимп для Android и измените свои стандарты в мире ставок! [url=https://apk-olimp.ru/]Скачать Olimp на андроид[/url] доступно для всех. С современным дизайном и ускоренной загрузкой данных, доступ к различным спортивным событиям становится игрой. Управление аккаунтом и навигация по приложению теперь настолько просты, что каждая ставка превращается в удовольствие. Установите последнюю версию прямо сейчас и начните добиваться успеха с БК Олимп, где каждый шаг может привести к значимому триумфу!
Всё о радиаторах отопления https://heat-komfort.ru/ – выбор радиатора, монтаж, обслуживание.
Недвижимость в Болгарии от застройщика и вторичная недвижимость https://bulgarianresales.com/3418/harmoni-syuits-zhilye-kompleksy
Visit the page https://t.me/melbet_1xbet_betwinner_promocode where you will find new, current 1xbet Betwinner Melbet | Promo code. The latest information on promotional codes that are valid now and throughout 2024.
На сайте https://chisto39.ru/ закажите профессиональную, качественную химчистку в тот же день, когда и обратились за услугой. На результат дается гарантия качества, потому как специалисты уверены в том, что услуга будет проведена именно так, как полагается и точно в обозначенные сроки. Среди основных услуг выделяют: химчистка ковров, диванов, матрасов, кресел, автомобилей. В работе используется только высокотехнологичное оборудование, качественная химия, которая избавляет от самых стойких пятен.
Откройте для себя новый горизонт ставок с обновленным приложением БК Олимп для Android! Переосмысленный дизайн и ускоренная обработка данных обеспечивают легкий доступ к широкому ассортименту спортивных событий. [url=https://app-olimb.ru/]Приложение букмекера Олимп[/url] для всех. Упрощенное управление счетом и легкость использования делают ставки приятным занятием. Установите это обновление немедленно и выходите на новый уровень в ставках с БК Олимп, где каждое событие предоставляет шанс на победу!
Откройте для себя перспективы ставок с обновленным приложением БК Олимп для Android! Обновленный дизайн и быстрая загрузка обеспечивают легкий доступ к широкому ассортименту спортивных событий. [url=https://app-olimb.ru/]Скачать приложение конторы Олимп[/url] для вас. Упрощенное управление счетом и легкость использования делают ставки удовольствием. Установите это приложение немедленно и повышайте свои шансы в ставках с БК Олимп, где каждое событие предоставляет шанс на победу!
Откройте для себя перспективы ставок с обновленным приложением БК Олимп для Android! Обновленный дизайн и ускоренная обработка данных обеспечивают легкий доступ к многообразию спортивных событий. [url=https://app-olimb.ru/]Olimp bet на телефон[/url] для каждого. Упрощенное управление счетом и интуитивная навигация делают ставки удовольствием. Загрузите это приложение сейчас же и выходите на новый уровень в ставках с БК Олимп, где каждое событие предоставляет шанс на победу!
Откройте для себя перспективы ставок с обновленным приложением БК Олимп для Android! Обновленный дизайн и ускоренная обработка данных обеспечивают легкий доступ к широкому ассортименту спортивных событий. [url=https://app-olimb.ru/]Скачать контору Олимп на андроид[/url] для всех. Простота ведения финансов и интуитивная навигация делают ставки приятным занятием. Загрузите это обновление сейчас же и выходите на новый уровень в ставках с БК Олимп, где каждое событие предоставляет шанс на успех!
Компания «Сомово» предлагает надежную мебель. Стремимся дать покупателям только самое лучшее. Наши спальни продуманы до мелочей, приобрести их вы можете у нас недорого с оперативной доставкой. Вся продукция сертифицирована. Ищете гостиные купить в москве? Somovo-mebel.ru – сайт, где можно отыскать множество вариантов комфортных спален. Заботимся о качестве товаров и своей репутации. Делаем все, чтобы вы покупкой были довольны. Рекомендуйте нас и обращайтесь снова!
מקצועית ויוצאת דופן. כאן תוכלו בקלות למצוא דירות דיסקרטיות בצפון, בתל אביב והמרכז ואזור הדרום. הדירות זמינות בכל שעות היממה ובכל ימות השבוע ותמיד אפשר לבוא וליהנות. הבילוי מתאים לכל הגברים, מהרווקים ועד הנשואים ושומרי המסורת. שירותי ליווי ברמה של חו“ל מכוני ליווי – לא מה שחשבת (או אולי כן?)
Зайдите на сайт https://yeezy-boost-shop.ru/ и купите по выгодной цене Кроссовки Adidas Yeezy Boost оригинал. Магазин Адидас Изи Буст это широкий ассортимент, а сами кроссовки это уникальный дизайн, инновационные материалы и непревзойденный комфорт. Кроссовки Yeezy разных моделей и размеров всегда в наличии. Подробнее на сайте.
בליאור סקס, ניתן למצוא מודעות המפרטות בחלקן מהם אותם שירותים. ניתן למצוא מידע על עיסויים ארוטיים, אוראליים, ואפילו אנאליים. ניתן למצוא מידע על עריכת מופעי חשפנות בהופעות ומסיבות. שנית, מומלץ לשבץ בכל מסיבת רווקים חשפנית איכותית, ולא לוותר על כך. זכרו שזה בחורות בקריות
הרגשה טובה יותר מלקבל עיסוי אירוטי מפנק על הבוקר ואין שינה יותר טובה מאשר לאחר הרפיה מלאה. מודעות של מגוון מעסות ארוטיות מקצועיות ולוהטות מחכות לך באתר, לך נשאר רק לבחור נערות ליווי פרטיות שיודעות להפוך כל ערב לחוויה נערות ליווי פרטיות מכל הסוגים Meet Jerusalem escort sexy girls
На сайте https://vitaminium.shop/ у вас есть возможность приобрести минеральные комплексы и витамины для всей семьи. Менеджеры компании предложат для вас самый высокий уровень сервиса, выгодные условия сотрудничества, большой ассортимент минералов и витаминов, а также компетентную консультацию. Оплата удобная, выберите товар, добавьте его в «Корзину» и оформите заказ. Доставка осуществляется в любую страну. Подробнее ознакомиться с акциями, каталогом и увидеть нашу контактную информацию, вы можете на сайте.
Stumbled upon a unique article, I suggest you take a look http://fire-team.ru/forum/member.php?u=1143
На сайте https://dk-polymer.com оформите заявку для того, чтобы воспользоваться такой услугой, как создание полимерных изделий. Они создаются на базе тех технологий, которые были разработаны с использованием инновационных исследований. Особенностью продукции является то, что она сертифицированная, полностью безопасная. На каждом этапе разработки осуществляется контроль качества. В компании есть свой автопарк, организуется логистика. Производство строго в обозначенные сроки.
строительство домов из бруса проекты [url=http://www.alexstroy53.ru]http://www.alexstroy53.ru[/url] .
Запускайте новую версию приложения БК Олимп для Android и переопределите свои стандарты в мире ставок! [url=https://apk-olimp.ru/]Приложение Олимп[/url] уже сегодня. С передовым дизайном и ускоренной загрузкой данных, доступ к различным спортивным событиям становится игрой. Ведение счета и навигация по приложению теперь так удобны, что каждая ставка превращается в удовольствие. Скачайте последнюю версию прямо сейчас и начните выигрывать с БК Олимп, где каждый шаг может привести к значимому триумфу!
Запускайте новую версию приложения БК Олимп для Android и измените свои стандарты в мире ставок! [url=https://apk-olimp.ru/]Olimp на андроид[/url] уже сегодня. С передовым дизайном и быстрой загрузкой данных, доступ к многочисленным спортивным событиям становится игрой. Управление аккаунтом и навигация по приложению теперь так удобны, что каждая ставка превращается в удовольствие. Скачайте последнюю версию без задержек и начните выигрывать с БК Олимп, где каждый шаг может привести к значимому триумфу!
Запускайте новую версию приложения БК Олимп для Android и переопределите свои стандарты в мире ставок! [url=https://apk-olimp.ru/]Olimp bet на андроид[/url] сегодня. С передовым дизайном и ускоренной загрузкой данных, доступ к различным спортивным событиям становится легкостью. Управление аккаунтом и навигация по приложению теперь настолько просты, что каждая ставка превращается в радость. Скачайте последнюю версию без задержек и начните выигрывать с БК Олимп, где каждый шаг может привести к большой победе!
Запускайте новую версию приложения БК Олимп для Android и измените свои стандарты в мире ставок! [url=https://apk-olimp.ru/]Скачать приложение Olimp bet[/url] прямо сейчас. С современным дизайном и ускоренной загрузкой данных, доступ к многочисленным спортивным событиям становится легкостью. Ведение счета и навигация по приложению теперь так удобны, что каждая ставка превращается в удовольствие. Установите последнюю версию прямо сейчас и начните выигрывать с БК Олимп, где каждый шаг может привести к большой победе!
Сервисный центр Samsung https://galaxy-techservice.com/ это профессиональный сервис по ремонту техники Самсунг в Москве. Качественный и быстрый ремонт техники по доступным ценам. Гарантия на все виды работ. Ремонт любой сложности. Обученные и профессиональные сотрудники помогут вам восстановить технику или гаджет. Подробнее на сайте.
Запускайте свежую версию приложения БК Олимп для Android и измените свои стандарты в мире ставок! [url=https://apk-olimp.ru/]Скачать Олимп бесплатно[/url] сегодня. С современным дизайном и ускоренной загрузкой данных, доступ к многочисленным спортивным событиям становится легкостью. Управление аккаунтом и перемещение по разделам теперь так удобны, что каждая ставка превращается в удовольствие. Скачайте последнюю версию прямо сейчас и начните добиваться успеха с БК Олимп, где каждый шаг может привести к значимому триумфу!
Запускайте свежую версию приложения БК Олимп для Android и измените свои стандарты в мире ставок! [url=https://apk-olimp.ru/]Скачать Olimp bet для андроид[/url] уже сейчас. С передовым дизайном и быстрой загрузкой данных, доступ к многочисленным спортивным событиям становится игрой. Управление аккаунтом и перемещение по разделам теперь настолько просты, что каждая ставка превращается в радость. Скачайте последнюю версию без задержек и начните выигрывать с БК Олимп, где каждый шаг может привести к большой победе!
Зайдите на сайт Chronolux https://poljot.pro/ и ознакомьтесь с большим каталогом модных часов для каждого. В нашем ассортименте представлены современные, уникальные наручные часы от различных марок. Вы обязательно сможете подобрать часы под свой бюджет и по параметрам, а также купить ремни для часов.
Запускайте новую версию приложения БК Олимп для Android и переопределите свои стандарты в мире ставок! [url=https://apk-olimp.ru/]Скачать приложение Олимп бет[/url] прямо сейчас. С передовым дизайном и ускоренной загрузкой данных, доступ к различным спортивным событиям становится игрой. Ведение счета и перемещение по разделам теперь настолько просты, что каждая ставка превращается в радость. Скачайте последнюю версию прямо сейчас и начните добиваться успеха с БК Олимп, где каждый шаг может привести к большой победе!
Сайт v8corp.ru предоставляет виды услуг по цифровизации и автоматизации процессов с применением программ 1С. Сотрудничая с нами, вы сфокусируетесь на своем бизнесе, мы разбираемся в 24 отраслях экономики. https://v8corp.ru/ – сайт, где можете подробнее ознакомиться с конфигурациями 1С. У нас гибкие тарифы. Подберем для вас лучшую конфигурацию продуктов 1С, которые подходят под вашу компанию. Оцените открывающиеся преимущества облачной Аренды 1С. Мы готовы стать надежным вашим помощником. Получите консультацию уже сейчас, обращайтесь!
Откройте для себя новый горизонт ставок с обновленным приложением БК Олимп для Android! Переосмысленный дизайн и ускоренная обработка данных обеспечивают легкий доступ к многообразию спортивных событий. [url=https://app-olimb.ru/]Скачать приложение БК Олимп[/url] для всех. Упрощенное управление счетом и легкость использования делают ставки приятным занятием. Установите это приложение немедленно и выходите на новый уровень в ставках с БК Олимп, где каждое событие предоставляет шанс на победу!
Откройте для себя перспективы ставок с обновленным приложением БК Олимп для Android! Обновленный дизайн и ускоренная обработка данных обеспечивают легкий доступ к многообразию спортивных событий. [url=https://app-olimb.ru/]Букмекерская контора Олимп на андроид[/url] для каждого. Простота ведения финансов и легкость использования делают ставки удовольствием. Установите это приложение сейчас же и выходите на новый уровень в ставках с БК Олимп, где каждое событие предоставляет шанс на успех!
Откройте для себя новый горизонт ставок с обновленным приложением БК Олимп для Android! Обновленный дизайн и быстрая загрузка обеспечивают легкий доступ к многообразию спортивных событий. [url=https://app-olimb.ru/]Скачать Олимп бесплатно[/url] для всех. Упрощенное управление счетом и интуитивная навигация делают ставки удовольствием. Загрузите это приложение сейчас же и выходите на новый уровень в ставках с БК Олимп, где каждое событие предоставляет шанс на успех!
Откройте для себя перспективы ставок с обновленным приложением БК Олимп для Android! Переосмысленный дизайн и ускоренная обработка данных обеспечивают легкий доступ к широкому ассортименту спортивных событий. [url=https://app-olimb.ru/]Скачать приложение БК Олимп[/url] для каждого. Простота ведения финансов и легкость использования делают ставки удовольствием. Установите это приложение немедленно и повышайте свои шансы в ставках с БК Олимп, где каждое событие предоставляет шанс на победу!
Хотите купить на dvd увлекательные сериалы? Serialexpress вам поможет в этом. Тут можете найти диски, которых нет в иных магазинах. Качество записи сериалов отличное, уверены, что вы останетесь довольны приобретением. Товары доступны по привлекательной цене, они хорошо упакованы и целые. Ищете купить сериал в красноярске? Serialexpress.ru – сайт, где имеется каталог. Здесь также можно ознакомиться с условиями оплаты. У нас действует накопительная система скидок. Качество доставки вас порадует. Все вопросы можно задать менеджерам по электронной почте.
לענות על הציפיות שלכם ובמגוון רחב של מובנים. הרי צפוי להיות מדובר בדירות מפוארות ומרשימות מאוד לעתים, דבר אשר יוכל להבטיח לכם שתוכלו ליהנות מחוויה מינית אשר תתקיים במקום בו תוכלו להגשים את כל הפנטזיות שלכם ובנוחות הרבה ביותר שרק ניתן להעלות על הדעת. Enjoy Tel Aviv sex with the most seductive liberated women
Сайт https://krasnodar.climateon.ru/ это возможность купить кондиционер с установкой в Краснодаре, купить сплит систему в Краснодаре, купить кондиционер в Краснодаре в широком ассортименте по выгодным ценам. Большой каталог товаров, в том числе кондиционеры Краснодар, позволят вам купить продукцию известных брендов. Подробнее на сайте.
Came across an interesting article, worth a glance https://www.0552.ua/list/472301
[url=https://valtrexmedication.online/]how to buy valtrex in korea[/url]
На сайте https://concept-digital.ru вам будет предложены услуги рекламного агентства. Разрабатываем стратегию, проводим анализ рынка и увеличиваем продажи. Студия рекламы предоставляет услуги дизайнера и маркетолога. Работая с нами, вы получите 100% гарантию на положительный результат. Подробнее ознакомиться с ценами и услугами рекламного агентства вы можете на сайте. Также здесь имеется портфолио. У нас заказывают, потому, как мы обладаем приличным опытом в продажах, имеем честные цены и правильно подходим к работе.
Сайт Demo-1c.ru поможет вам узнать, кому будет полезна аренда 1С. К вашим услугам демоверсии приложений 1С. Главные достоинства применения облачного сервиса «1С Демо» и «Аренда 1С»: каждодневное резервное копирование данных, удаленное сопровождение программ «1С», также круглосуточная техническая поддержка. https://demo-1c.ru/ – сайт, где можно увидеть перед арендой, покупкой распространенные решения 1С. У нас только проверенные опытом профессионалы. Закажите грамотную консультацию прямо сейчас!
Компания «КапиталСтройАльянс» специализируется на производстве окон, дверей из ПВХ и алюминия. В своей сфере она имеет отменную репутацию. Качество нашей продукции мы довели до идеала. Гарантируем высокую степень точности изделия с отсутствием брака и быстрые сроки изготовления. Лучшие мастера быстро поставят окна и оставят после себя порядок. http://oknaksa.ru – сайт, где у вас есть возможность ознакомиться с отзывами и осуществленными работами. Если будет нужно, мы вас проконсультируем. Всегда рады видеть вас!
[url=https://tadalafilu.online/]tadalafil 20mg[/url]
משתוקקים בכל זאת לתירוץ טוב שיאפשר לנו להיעלם לכמה שעות או לערב שלם, לבלות עם בחורה סקסית שתשכיח מאיתנו את הצרות, וליהנות כמו שצריך. בין אם התירוץ שלכם להזמין נערת ליווי הוא אירוח של לקוח חשוב, תכנון מסיבת רווקים לחבר הכי טוב או ערב שקט שבו זכיתם כשהאישה נערות ליווי בירושלים
דירות דיסקרטיות באזורך הדירה הדיסקרטית שלך ממתינה לך בכל יום ובכל שעה. מרבית המקומות פועלים עשרים וארבע שעות ביממה וניתן להגיע באמצע הלילה או בהפסקת הצהריים. בין אם מדובר בתחילת היום או בשעות הקטנות של הלילה, הדירה מחכה לך נקייה ומוכנה אך ורק עבורך בכדי Meet hot Independent escort Tel Aviv girls
Хотите смотреть онлайн достойные фильмы и скачивать их в отменном качестве бесплатно? Tartugi в этом вам, несомненно, поможет! Мы собрали наилучший контент, который вас порадует. Садитесь поудобнее и окунитесь в мир непревзойденных историй. https://good.tartugi.net – известный портал, где найдете все, что вам необходимо. Сайт имеет удобный интерфейс, разобраться с ним очень просто. Всегда в доступе интересные фильмы и сериалы онлайн, вы их можете скачать через торрент. Tartugi всегда рад вам!
На сайте https://shemi-otopleniya.ru/ почитайте информацию, которая касается гибкой подводки, выполненной из нержавеющей стали. Есть конструкции различной модификации и размера, а потому не составит труда выбрать наиболее подходящий под ваши запросы вариант. Есть информация и о том, как правильно проводить монтаж, в каких случаях используется гибкая подводка и многое другое. Также опубликованы фотографии для большей наглядности. Вся информация является качественной, актуальной, а потому на нее можно ориентироваться.
[url=http://oazithromycin.com/]azithromycin coupon[/url]
вилла на кипре купить первая линия
bitcoin balance checker software
[url=http://oprednisone.online/]prednisone 60mg daily[/url]
Understanding COSC Accreditation and Its Importance in Horology
COSC Accreditation and its Demanding Criteria
Controle Officiel Suisse des Chronometres, or the Controle Officiel Suisse des Chronometres, is the authorized Swiss testing agency that certifies the precision and precision of wristwatches. COSC accreditation is a mark of excellent craftsmanship and trustworthiness in chronometry. Not all timepiece brands follow COSC accreditation, such as Hublot, which instead follows to its proprietary strict criteria with mechanisms like the UNICO calibre, attaining comparable precision.
The Science of Exact Timekeeping
The core system of a mechanized watch involves the spring, which delivers energy as it unwinds. This mechanism, however, can be susceptible to environmental elements that may impact its precision. COSC-accredited mechanisms undergo demanding testing—over fifteen days in various conditions (5 positions, 3 temperatures)—to ensure their durability and reliability. The tests measure:
Typical daily rate accuracy between -4 and +6 seconds.
Mean variation, highest variation levels, and impacts of temperature variations.
Why COSC Validation Matters
For timepiece fans and collectors, a COSC-accredited watch isn’t just a item of tech but a testament to lasting quality and accuracy. It represents a timepiece that:
Offers exceptional reliability and precision.
Ensures confidence of quality across the entire construction of the watch.
Is apt to hold its worth more efficiently, making it a wise investment.
Famous Chronometer Manufacturers
Several well-known brands prioritize COSC validation for their watches, including Rolex, Omega, Breitling, and Longines, among others. Longines, for instance, offers collections like the Archive and Soul, which feature COSC-validated mechanisms equipped with advanced substances like silicone balance springs to improve durability and performance.
Historical Background and the Development of Chronometers
The notion of the chronometer originates back to the requirement for exact timekeeping for navigation at sea, emphasized by John Harrison’s work in the 18th century. Since the official establishment of Controle Officiel Suisse des Chronometres in 1973, the validation has become a standard for evaluating the accuracy of high-end watches, sustaining a tradition of superiority in horology.
Conclusion
Owning a COSC-certified watch is more than an visual choice; it’s a commitment to excellence and accuracy. For those appreciating precision above all, the COSC validation provides peace of thoughts, guaranteeing that each validated timepiece will operate dependably under various circumstances. Whether for personal satisfaction or as an investment decision, COSC-accredited watches stand out in the world of horology, carrying on a legacy of meticulous timekeeping.
Вам необходима психологическая помощь? Грудинин Александр Михайлович – компетентный врач-психотерапевт, который внимательно вас выслушает, с ним разговаривать будет очень комфортно. Он выявит и устранит ваши внутренние психологические проблемы. http://grudinin-psihoterapevt.ru – сайт, где имеется детальная информация о специалисте, посмотрите ее уже сегодня. Также тут есть статьи, тесты и постоянно задаваемые вопросы. Можно записаться на прием к доктору, позвонив по контактному номеру телефона.
На сайте https://stranauslug.by/ ознакомьтесь с частными объявлениями от самых разных мастеров и различного направления. Здесь вы сможете заказать услуги, связанные со строительством, отделкой помещения, а также грузоперевозки. Кроме того, есть возможность записаться на йогу, в фитнес-клуб, зумбу или бальные танцы. Регулярно появляются новые объявления с предложениями, что позволит отыскать подходящий вариант по привлекательной стоимости. Здесь же получится арендовать автомобиль, спецтехнику.
Если желаете купить интересные сериалы на DVD, то Serialexpress может предложить вам такую возможность. Запись сериалов довольно качественная, вы точно останетесь довольны покупкой. Товары прекрасно упакованы, цены приемлемые. Ищете купить документальные сериалы? Serialexpress.ru – сайт, где можно подробно ознакомиться с каталогом и выгодными предложениями. Также тут указаны условия доставки и оплаты. Появились какие-либо вопросы? Задайте их по электронной почте, которая указана на нашем сервисе. Менеджеры вежливые, они с радостью готовы помочь вам. Обращайтесь!
שהגיעה השעה לחגוג עמן. כל המעוניין בזמן איכות ובהתפרקות מלחצי השגרה יכול להתפנק בעיסוי ארוטי, להשתמש או להיפגש עם הנערות בחדר בית המלון. הן יהיו זמינות עבורכם למשך כל שעות היממה. מומלץ במיוחד עבור לקוחות כמו אוליגרכים ובעלי מבאים כאלו ואחרים. אם אתם זונות להזמנה
резиновая баба сколько стоит
На сайте https://shemi-otopleniya.jimdo.com/ вы сможете получить всю необходимую информацию, которая касается гибкой подводки из нержавеющей стали. Она может быть самого разного размера, диаметра, длины, что позволит подобрать вариант в соответствии с нуждами, целями, а также предпочтениями. Также вы узнаете и о том, как правильно проводить монтажные работы, чтобы продлить эксплуатационные сроки. Все изделия выполнены из надежных, качественных материалов, которые сохранят свой вид надолго.
На сайте https://www.youtube.com/channel/UCXvwfNDluFJ-oeq9DKSapeQ вы сможете увидеть обновленную версию Прочных связей «MARVEL Человек-Паук», которая позволяет сыграть за опытного Питера Паркера. Он борется со злодеями и крупной преступностью в Нью-Йорке, постоянно балансирует на грани, пытаясь одновременно построить карьеру, разобраться в любви и защитить жителей. Плейлист приложение найдете на ютубе. Подписывайтесь на канал и ставьте лайки, а подробное описание вы найдете на канале, где также активно можно давать свои комментарии.
חוד החנית של כל אירוע רווקים! מדוע זה כך? משום שזה יוצר אווירה חמה ואיכותית בה כל גבר חש את הערך המוסף של האירוע. חשוב מכל: ההגעה של החשפנית יכולה להיעשות גם בהתראה קצרה, ולהגיע למקום בתוך דקות בודדות נערת הליווי באזורך להזמנה עם תמונות אמיתיות לביתך דירות דיסקרטיות בדרום
ModaOK предлагает вам окунуться в мир стиля и моды. Здесь имеются интересные статьи о моде, татуировках, прическах и др. У нас имеется лунный календарь стрижек. Также тут мы делимся полезными советами. Ищете окрашивание омбре фото на короткие волосы? Modaok.ru – сайт, который предназначен для тех, кто хочет быть в тренде. Здесь вы узнаете, с чем носить вельветовые брюки, почему молодожены выбирают обручальные кольца с гравировкой и др. Регулярно обновляем информацию, чтобы вы могли быть в курсе последних модных тенденций. Не упустите возможность стать стильной вместе с ModaOK!
Came across an interesting article, worth a glance http://or1gano.80lvl.ru/viewtopic.php?f=10&t=872
На сайте https://iz-nerzhaveyki.ru/ почитайте информацию, которая связана с фитингами, кранами, подводками, выполненными из нержавеющей стали, шаровыми кранами, а также фильтрами, обратными клапанами, муфтами, арматурой, прокатом. Перед вами гибкая подводка самых разных размеров, модификаций, но вся она наделена длительным сроком эксплуатации, высоким качеством, на ней не образуется коррозия. Все конструкции выполнены по ГОСТу, в соответствии с самыми жесткими требованиями. Отличаются небольшой стоимостью.
На сайте https://egorkosarev.ru/ воспользуйтесь услугами видеооператора и видеомонтажера из Москвы. Он будет вам полезен, если планируете снять рекламное, детское видео, а также художественное или музыкальное. Также возможна и съемка интервью. Для того чтобы получить представление о работе, посмотрите шоурум. Огромное количество работ в портфолио. Все услуги оказываются по привлекательной стоимости и тогда, когда вам необходимо. В обязательном порядке обсуждается проект, заключается договор.
Приветствуем вас в мир ставок с БК Лига Ставок! Процесс регистрации и входа в личный кабинет созданы для обеспечения простоты и безопасности каждого пользователя. [url=https://ligastavok-login.ru/]ligastavok-login.ru[/url] – легко и быстро, и это открывает доступ к многочисленным возможностям: от ставок на спорт до участия в акциях и получения бонусов.
Пройдите несколько простых шагов для регистрации: заполните обязательные поля формы, подтвердите свою электронную почту и номер телефона, и вы на пути к успеху! После регистрации, войдите в личный кабинет с помощью полученных данных – и весь мир ставок БК Лига Ставок окажется у вас под рукой.
Ваш личный кабинет – это центр управления вашими ставками, где вы можете легко управлять своим балансом, участвовать в играх, просматривать историю транзакций, а также получать эксклюзивные предложения и уведомления о предстоящих событиях. Не теряйте времени – присоединяйтесь к БК Лига Ставок уже сегодня и начните своё путешествие в мире ставок с комфортом и уверенностью!
Приветствуем вас в мир ставок с БК Лига Ставок! Процесс регистрации и входа в личный кабинет созданы для максимального удобства и безопасности каждого пользователя. [url=https://ligastavok-login.ru/]Вход в лига ставок[/url] – просто и удобно, и это открывает доступ к широкому спектру функций: от ставок на спорт до участия в акциях и получения бонусов.
Пройдите несколько простых шагов для регистрации: заполните обязательные поля формы, подтвердите свою электронную почту и номер телефона, и вы готовы к старту! После регистрации, войдите в личный кабинет с помощью полученных данных – и весь мир ставок БК Лига Ставок окажется у вас под рукой.
Ваш личный кабинет – это центр управления вашими ставками, где вы можете легко пополнять счет, участвовать в играх, просматривать историю транзакций, а также получать эксклюзивные предложения и уведомления о предстоящих событиях. Не упустите свой шанс – присоединяйтесь к БК Лига Ставок уже сегодня и погрузитесь в мир ставок с комфортом и уверенностью!
Приветствуем вас в мир ставок с БК Лига Ставок! Процесс регистрации и входа в личный кабинет созданы для обеспечения простоты и безопасности каждого пользователя. [url=https://ligastavok-login.ru/]Лига ставок регистрация с бонусом[/url] – легко и быстро, и это открывает доступ к многочисленным возможностям: от ставок на спорт до участия в акциях и получения бонусов.
Начните с нескольких легких шагов для регистрации: заполните обязательные поля формы, подтвердите свою электронную почту и номер телефона, и вы на пути к успеху! После регистрации, используйте ваш новый аккаунт с помощью полученных данных – и весь мир ставок БК Лига Ставок окажется у вас под рукой.
Ваш личный кабинет – это центр управления вашими ставками, где вы можете легко управлять своим балансом, участвовать в играх, просматривать историю транзакций, а также получать эксклюзивные предложения и уведомления о предстоящих событиях. Не теряйте времени – присоединяйтесь к БК Лига Ставок уже сегодня и погрузитесь в мир ставок с комфортом и уверенностью!
Добро пожаловать в мир ставок с БК Лига Ставок! Процесс регистрации и входа в личный кабинет созданы для максимального удобства и безопасности каждого пользователя. [url=https://ligastavok-login.ru/]Лига ставок официальный сайт регистрация[/url] – просто и удобно, и это открывает доступ к многочисленным возможностям: от ставок на спорт до участия в акциях и получения бонусов.
Пройдите несколько простых шагов для регистрации: заполните обязательные поля формы, подтвердите свою электронную почту и номер телефона, и вы на пути к успеху! После регистрации, используйте ваш новый аккаунт с помощью полученных данных – и весь мир ставок БК Лига Ставок окажется у вас под рукой.
Ваш личный кабинет – это командный пункт вашими ставками, где вы можете легко управлять своим балансом, делать ставки, просматривать историю транзакций, а также получать эксклюзивные предложения и уведомления о предстоящих событиях. Не теряйте времени – присоединяйтесь к БК Лига Ставок уже сегодня и погрузитесь в мир ставок с комфортом и уверенностью!
Приветствуем вас в мир ставок с БК Лига Ставок! Процесс регистрации и входа в личный кабинет созданы для максимального удобства и безопасности каждого пользователя. [url=https://ligastavok-login.ru/]Лига ставок регистрация через госуслуги[/url] – легко и быстро, и это открывает доступ к многочисленным возможностям: от ставок на спорт до участия в акциях и получения бонусов.
Пройдите несколько простых шагов для регистрации: заполните обязательные поля формы, подтвердите свою электронную почту и номер телефона, и вы на пути к успеху! После регистрации, используйте ваш новый аккаунт с помощью полученных данных – и весь мир ставок БК Лига Ставок окажется у вас под рукой.
Ваш личный кабинет – это командный пункт вашими ставками, где вы можете легко пополнять счет, участвовать в играх, просматривать историю транзакций, а также получать эксклюзивные предложения и уведомления о предстоящих событиях. Не теряйте времени – присоединяйтесь к БК Лига Ставок уже сегодня и начните своё путешествие в мире ставок с комфортом и уверенностью!
Приветствуем вас в мир ставок с БК Лига Ставок! Процесс регистрации и входа в личный кабинет созданы для максимального удобства и безопасности каждого пользователя. [url=https://ligastavok-login.ru/]Лига ставок регистрация фрибет[/url] – легко и быстро, и это открывает доступ к широкому спектру функций: от ставок на спорт до участия в акциях и получения бонусов.
Пройдите несколько простых шагов для регистрации: заполните обязательные поля формы, подтвердите свою электронную почту и номер телефона, и вы готовы к старту! После регистрации, используйте ваш новый аккаунт с помощью полученных данных – и весь мир ставок БК Лига Ставок окажется у вас под рукой.
Ваш личный кабинет – это центр управления вашими ставками, где вы можете легко управлять своим балансом, участвовать в играх, просматривать историю транзакций, а также получать эксклюзивные предложения и уведомления о предстоящих событиях. Не упустите свой шанс – присоединяйтесь к БК Лига Ставок уже сегодня и начните своё путешествие в мире ставок с комфортом и уверенностью!
Приветствуем вас в мир ставок с БК Лига Ставок! Процесс регистрации и входа в личный кабинет созданы для обеспечения простоты и безопасности каждого пользователя. [url=https://ligastavok-login.ru/]ligastavok-login.ru[/url] – легко и быстро, и это открывает доступ к многочисленным возможностям: от ставок на спорт до участия в акциях и получения бонусов.
Пройдите несколько простых шагов для регистрации: заполните обязательные поля формы, подтвердите свою электронную почту и номер телефона, и вы на пути к успеху! После регистрации, войдите в личный кабинет с помощью полученных данных – и весь мир ставок БК Лига Ставок окажется у вас под рукой.
Ваш личный кабинет – это командный пункт вашими ставками, где вы можете легко пополнять счет, делать ставки, просматривать историю транзакций, а также получать эксклюзивные предложения и уведомления о предстоящих событиях. Не теряйте времени – присоединяйтесь к БК Лига Ставок уже сегодня и погрузитесь в мир ставок с комфортом и уверенностью!
vovan casino официальный сайт
Международный институт современного образования достойный внимания, он осуществляет профессиональную переподготовку специалистов. Преподаватели – настоящие профессионалы. Процесс учебы поставлен на высоком уровне. Ищете профессиональное обучение? Misokmv.ru – сайт, где представлена более подробная информация о нас. Мы гарантируем внимательное отношение и считаем, что учиться никогда не поздно. Вы всегда можете получить ответы на свои вопросы. Выбирайте нас и не пожалеете об этом. Получить образование в «МИСО» – удобно и выгодно, звоните нам прямо сейчас!
Хотите купить семена высокого качества? Семена на Яблочкова онлайн-магазин – это то, что вам необходимо. Здесь можно отыскать семена цветов, овощей, трав и иных растений. Мы постоянно обновляем наш ассортимент, гарантируем удобство заказа, квалифицированную поддержку и высочайшее качество. Ищете семена огурцов купить? Magazinsemena.ru – сайт, где имеется широкий выбор семян различных растений для наших клиентов. Предоставляем надежную и быструю доставку. Готовы ответить на все ваши вопросы и помочь с выбором.
Какие радиаторы выбрать для отопления https://propest.ru/kak-vybrat-radiator-dlya-doma.html частного загородного дома – каменного или деревянного: разновидности и классы, правила выбора, цены, сравнения.
На сайте https://smotret.net/ вы сможете найти все самые интересные, увлекательные сериалы на самый взыскательный вкус. Они доступны для просмотра не только на ПК, но и смартфоне, планшете. Подберите вариант по жанру, году выпуска, стране. Есть легендарные фильмы, которые стали модные еще в начале 2000-х, но также есть и новинки, которые точно никого не оставят равнодушным. Сериалы, фильмы на самую разную тематику. Смотрите их в любое время, абсолютно бесплатно. Перед вами огромный выбор вариантов, которые можно посмотреть вечером.
Рады представить выход новой версии мобильного приложения БК Лига Ставок для Android! Этот шаг существенно улучшает ваш пользовательский опыт, делая его ещё удобнее и эффективным. [url=https://ligastavok-android.ru/]Приложение лига ставок скачать[/url] сейчас же! В обновлении вы найдете свободу доступа к огромному ассортименту спортивных мероприятий прямо с вашего смартфона. Усовершенствованное управление профилем, инновационная концепция дизайна для простой навигации и значительно более высокая скорость работы приложения – всё это создано для вас. Станьте частью воодушевленных пользователей и наслаждайтесь возможностями от ставок в любой точке планеты и в любой момент. Скачайте обновление приложения БК Лига Ставок уже сегодня и переходите к новому этапу ставок!
Анонсируем выход новой версии мобильного приложения БК Лига Ставок для Android! Этот выпуск полностью преобразует ваш досуг с ставками, делая его более понятным и динамичным. [url=https://ligastavok-android.ru/]Бесплатно скачать лига ставок[/url] для всех пользователей! В обновлении вы найдете свободу доступа к огромному ассортименту спортивных мероприятий из любой точки с помощью вашего устройства. Улучшенное управление профилем, инновационная концепция дизайна для простой навигации и значительно более высокая скорость работы приложения – всё это создано для вас. Станьте частью воодушевленных пользователей и наслаждайтесь возможностями от ставок в любой точке планеты и в любой момент. Установите обновление приложения БК Лига Ставок уже сегодня и выходите на новый уровень ставок!
Анонсируем выход обновленной мобильного приложения БК Лига Ставок для Android! Этот выпуск полностью преобразует ваш досуг с ставками, делая его более понятным и динамичным. [url=https://ligastavok-android.ru/]Скачать бк лига ставок на телефон[/url] немедленно! В обновлении вы найдете свободу доступа к огромному ассортименту спортивных мероприятий из любой точки с помощью вашего устройства. Улучшенное управление профилем, передовая концепция дизайна для удобной навигации и значительно более высокая скорость работы приложения – всё это создано для вас. Присоединяйтесь к числу воодушевленных пользователей и наслаждайтесь возможностями от ставок где только захотите и в любой момент. Установите обновление приложения БК Лига Ставок уже сегодня и выходите на новый уровень ставок!
Рады представить выход новой версии мобильного приложения БК Лига Ставок для Android! Этот выпуск существенно улучшает ваш пользовательский опыт, делая его ещё удобнее и динамичным. [url=https://ligastavok-android.ru/]Скачать лига ставок на андроид apk[/url] для всех пользователей! В обновлении вы найдете прямой доступ к многообразию спортивных мероприятий прямо с вашего смартфона. Улучшенное управление профилем, передовая концепция дизайна для удобной навигации и значительно более высокая скорость работы приложения – всё это создано для вас. Присоединяйтесь к числу воодушевленных пользователей и получайте удовольствие от ставок в любой точке планеты и в любой момент. Скачайте обновление приложения БК Лига Ставок уже сегодня и переходите к новому этапу ставок!
Анонсируем выход обновленной мобильного приложения БК Лига Ставок для Android! Этот шаг существенно улучшает ваш досуг с ставками, делая его более понятным и динамичным. [url=https://ligastavok-android.ru/]Бк лига ставок официальный сайт скачать[/url] уже сегодня! В обновлении вы найдете прямой доступ к многообразию спортивных мероприятий прямо с вашего смартфона. Усовершенствованное управление профилем, инновационная концепция дизайна для удобной навигации и значительно более высокая скорость работы приложения – всё это создано для вас. Присоединяйтесь к числу воодушевленных пользователей и получайте удовольствие от ставок где только захотите и в любой момент. Скачайте обновление приложения БК Лига Ставок уже сегодня и переходите к новому этапу игры!
На сайте коллегии юристов http://zpp-1.ru/ вы найдете контакты и сможете связаться с адвокатами. Юрист расскажет о том, как нужно правильно поступить, поможет собрать необходимые документы и будет защищать ваши права в суде. Квалифицированная юридическая и медицинская поддержка призывникам с гарантией!
На сайте https://de.wikibrief.org/ вы сможете ознакомиться с интересной информацией на самую разную тему, включая радугу, время, население земного шара, золотой стандарт, всемирные банки и многое другое. Вся информация интересная, познавательная, а потому освещает все интересные и самые значимые вопросы. Все статьи написаны специалистами в данном вопросе, а потому данные подлинные, интересные и расширят ваш кругозор. Постоянно на сайте публикуются новые статьи, которые тоже будут вам интересны. Они касаются самых разных сфер.
Узнайте подробнее о выборе машины тут
בעזרת הידיים המיומנות והגוף המרגיע של מעסות מקצועיות. אין דבר יותר כיף מלקבל עיסוי ארוטי מפנק על הבוקר אך העיסוי יכול גם להכין אותך לקראת יציאה גדולה בערב או לשלוח אותך לישון בדרך שתבטיח חלומות טובים. אנחנו מזמינים אותך לנצל את המאגר הרחב שאספנו עבורך Tel Aviv escort girls for pleasing the pickiest men
Хотите провести незабываемый отдых в Кисловодске? Тогда вам нужно заранее забронировать жилье. Сделайте это прямо сейчас и наслаждайтесь отпуском. Выбирайте тот отель, который именно вам подходит по стоимости и расположению. https://kislovodsk-oteli.ru – сайт, над которым мы постоянно работаем, чтобы вам было комфортно с ним. Здесь имеется вся полезная информация для туристов. Тут вы узнаете, где именно забронировать идеальный номер. Рекомендуем вам обратить внимание на Goldyssun, отель гарантирует комфорт для абсолютно каждого гостя!
Зайдите на сайт Лишь Вам https://lishvam.ru/ который является электронной площадкой для размещения товаров и услуг. Для покупателей ассортимент более чем из 100.000 товаров, а доставка осуществляется по всей России. Ознакомьтесь с каталогом и выбирайте товары по выгодным ценам, получайте бонусы за покупки.
Opened up an enthralling read – I’d like to share it with you https://oldforum.citysakh.ru/?talkid=26104
Знакомьтесь с эксклюзивное предложение от БК Лига Ставок – фрибет для новых игроков! Это бесплатная ставка позволяет вам сделать ставку без риска потери собственных средств, открывая широкие возможности для выигрыша с первых же шагов в мире ставок. [url=https://ligastavok-freebet.ru/]Лига ставок фрибет 500[/url] для всех новичков после регистрации! С фрибетом от БК Лига Ставок, вы получаете шанс испытать все преимущества ставок без каких-либо финансовых обязательств. Это отличный способ начать для тех, кто хочет стать частью мира спортивных ставок с минимальными рисками. Не упускайте свой шанс и сделайте первый шаг к большим выигрышам в БК Лига Ставок с нашим фрибетом. Заберите свой фрибет уже сегодня и стартуйте к выигрышам!
Вашему вниманию предлагается эксклюзивное предложение от БК Лига Ставок – фрибет для новых игроков! Это бесплатная ставка даёт возможность вам испытать удачу без риска потери собственных средств, предоставляя новые горизонты для получения призов с первых же шагов в мире ставок. [url=https://ligastavok-freebet.ru/]Фрибет за установку приложения лига ставок[/url] для всех новичков после регистрации! С фрибетом от БК Лига Ставок, вы имеете возможность испытать все преимущества ставок без каких-либо финансовых обязательств. Это идеальный старт для тех, кто хочет погрузиться в мир ставок с минимальными рисками. Не упускайте свой шанс и сделайте первый шаг к большим выигрышам в БК Лига Ставок с нашим фрибетом. Активируйте ваш фрибет сейчас же и стартуйте к выигрышам!
Вашему вниманию предлагается эксклюзивное предложение от БК Лига Ставок – фрибет для новых игроков! Это фрибет даёт возможность вам испытать удачу без риска потери собственных средств, предоставляя новые горизонты для выигрыша с первых же шагов в мире ставок. [url=https://ligastavok-freebet.ru/]Фрибет лига ставок колесо фортуны[/url] новым пользователям после регистрации! С фрибетом от БК Лига Ставок, вы имеете возможность ознакомиться с платформой без каких-либо финансовых обязательств. Это отличный способ начать для тех, кто хочет стать частью мира спортивных ставок с минимальными рисками. Не упускайте свой шанс и начните своё приключение в БК Лига Ставок с нашим фрибетом. Активируйте ваш фрибет уже сегодня и откройте для себя мир ставок без рисков!
Знакомьтесь с эксклюзивное предложение от БК Лига Ставок – фрибет для новых игроков! Это бесплатная ставка даёт возможность вам испытать удачу без риска потери собственных средств, предоставляя новые горизонты для выигрыша с первых же шагов в мире ставок. [url=https://ligastavok-freebet.ru/]ligastavok-freebet.ru[/url] новым пользователям после регистрации! С фрибетом от БК Лига Ставок, вы получаете шанс ознакомиться с платформой без каких-либо финансовых обязательств. Это идеальный старт для тех, кто хочет стать частью мира спортивных ставок с минимальными рисками. Не упускайте свой шанс и сделайте первый шаг к большим выигрышам в БК Лига Ставок с нашим фрибетом. Активируйте ваш фрибет уже сегодня и стартуйте к выигрышам!
Знакомьтесь с эксклюзивное предложение от БК Лига Ставок – фрибет для новых игроков! Это бесплатная ставка даёт возможность вам испытать удачу без риска потери собственных средств, открывая широкие возможности для выигрыша с первых же шагов в мире ставок. [url=https://ligastavok-freebet.ru/]Лигаставок фрибет[/url] новым пользователям после регистрации! С фрибетом от БК Лига Ставок, вы имеете возможность ознакомиться с платформой без каких-либо финансовых обязательств. Это идеальный старт для тех, кто хочет стать частью мира спортивных ставок с минимальными рисками. Воспользуйтесь этим предложением и сделайте первый шаг к большим выигрышам в БК Лига Ставок с нашим фрибетом. Заберите свой фрибет сейчас же и откройте для себя мир ставок без рисков!
Вашему вниманию предлагается эксклюзивное предложение от БК Лига Ставок – фрибет для новых игроков! Это бесплатная ставка даёт возможность вам сделать ставку без риска потери собственных средств, открывая широкие возможности для получения призов с первых же шагов в мире ставок. [url=https://ligastavok-freebet.ru/]Лига ставок фрибет на день рождения[/url] новым пользователям после регистрации! С фрибетом от БК Лига Ставок, вы имеете возможность испытать все преимущества ставок без каких-либо финансовых обязательств. Это отличный способ начать для тех, кто хочет погрузиться в мир ставок с минимальными рисками. Воспользуйтесь этим предложением и начните своё приключение в БК Лига Ставок с нашим фрибетом. Заберите свой фрибет уже сегодня и откройте для себя мир ставок без рисков!
Вашему вниманию предлагается эксклюзивное предложение от БК Лига Ставок – фрибет для новых игроков! Это бесплатная ставка даёт возможность вам испытать удачу без риска потери собственных средств, предоставляя широкие возможности для выигрыша с первых же шагов в мире ставок. [url=https://ligastavok-freebet.ru/]Фрибет лига ставок за регистрацию[/url] для всех новичков после регистрации! С фрибетом от БК Лига Ставок, вы получаете шанс ознакомиться с платформой без каких-либо финансовых обязательств. Это отличный способ начать для тех, кто хочет стать частью мира спортивных ставок с минимальными рисками. Не упускайте свой шанс и сделайте первый шаг к большим выигрышам в БК Лига Ставок с нашим фрибетом. Заберите свой фрибет сейчас же и откройте для себя мир ставок без рисков!
На сайте https://muzzilka.ru/ послушайте качественную, яркую и приятную музыку на любой вкус и от лидирующих исполнителей. Она точно понравится вам своим звучанием, аранжировкой. Перед вами огромный выбор композиций, которые подарят положительные, яркие эмоции, настроят на позитив и помогут расслабиться. Кроме того, композиции помогут вернуться в прошлое и поностальгировать. Регулярно на портале появляются новинки, которыми сможет насладиться каждый. Слушайте музыку онлайн либо скачивайте на устройство.
Вам необходима профессиональная психологическая помощь? Тогда обратитесь в центр «Доверие». Специалисты окажут нужную поддержку, им важно ваше хорошее состояние. Вы научитесь понимать себя и разбираться в особенностях своей психики. Изменить свою жизнь к лучшему – вполне возможно. Требуется консультация психолога? Ack-group.ru – сайт, где можно записаться на прием к ответственному и компетентному психологу в любое время. Также здесь имеется актуальная цена консультации опытного специалиста. Свяжитесь с нами прямо сейчас. Не доводите ситуацию до предела!
Геоинтеллект – платформа, которая обладает множеством необходимых функций для сбора информации о населении, розничных конкурентах и др. Наши специалисты имеют достойный опыт работы аналитики геоданных. Они применяют лучшие научные практики, такие как: экономика, статистика, социальное и городское планирование. https://geointellect.com – сайт, где у вас есть возможность уже сегодня подробнее узнать о кейсах геомаркетинга. У нас имеется много удачных проектов во всех городах РФ, Узбекистана и Казахстана для сотен клиентов. Мы работаем для вас!
На сайте https://penza-evakuator.ru/ воспользуйтесь услугами легкового, грузового эвакуатора. В компании вас ожидают небольшие расценки – от 2 500 рублей. Оплатить услугу вы сможете любым, наиболее комфортным для себя способом. Работа происходит в круглосуточном режиме. Также можно воспользоваться и такими услугами, как: эвакуатор спецтехники, междугородняя эвакуация. Техника подается за считанные минуты, даже несмотря на ваше местоположение. Вас ожидает привлекательная стоимость.
Представляем выпуске эксклюзивного промокода для БК Лига Ставок! Этот специальный промокод предоставляет доступ к особым бонусам и повышает ваш опыт ставок, делая его более прибыльным и волнующим. [url=https://ligastavok-promocode.ru/]Лига ставок промокод за регистрацию[/url] для активации сегодня! С данным промокодом, игроки получают непосредственный доступ к бонусным средствам, ускоренной процедуре регистрации и специальным предложениям. Воспользуйтесь этой возможностью стать частью элитного клуба пользователей БК Лига Ставок и максимизируйте свои выигрыши с нашим промокодом. Используйте код прямо сейчас и начните выигрывать еще до первой ставки!
Рады сообщить о выпуске эксклюзивного промокода для БК Лига Ставок! Этот специальный промокод открывает доступ к эксклюзивным привилегиям и усиливает ваш опыт ставок, делая его более прибыльным и захватывающим. [url=https://ligastavok-promocode.ru/]Лигаставок промокод[/url] для всех новых пользователей! С его использованием, игроки получают доступ к дополнительным бонусам на ставки, ускоренной процедуре регистрации и специальным предложениям. Не упустите шанс стать частью привилегированной группы пользователей БК Лига Ставок и максимизируйте свои выигрыши с нашим промокодом. Используйте код уже сегодня и начните выигрывать еще до первой ставки!
Представляем доступности эксклюзивного промокода для БК Лига Ставок! Этот специальный промокод открывает доступ к особым бонусам и усиливает ваш опыт ставок, делая его более прибыльным и волнующим. [url=https://ligastavok-promocode.ru/]Промокод лига ставок на сегодня при регистрации[/url] сразу после активации! С данным промокодом, игроки получают непосредственный доступ к бонусным средствам, ускоренной процедуре регистрации и специальным предложениям. Воспользуйтесь этой возможностью стать частью элитного клуба пользователей БК Лига Ставок и максимизируйте свои выигрыши с нашим промокодом. Активируйте промокод прямо сейчас и заберите свои бонусы еще до первой ставки!
Представляем выпуске эксклюзивного промокода для БК Лига Ставок! Этот уникальный код предоставляет доступ к эксклюзивным привилегиям и усиливает ваш игровой процесс, делая его ещё более выгодным и волнующим. [url=https://ligastavok-promocode.ru/]Лига ставок бонус при регистрации промокод[/url] для активации сегодня! С его использованием, игроки получают непосредственный доступ к бонусным средствам, привилегиям при регистрации и эксклюзивным акциям. Не упустите шанс стать частью привилегированной группы пользователей БК Лига Ставок и увеличьте свои шансы на успех с нашим промокодом. Активируйте промокод прямо сейчас и начните выигрывать еще до первой ставки!
Представляем выпуске эксклюзивного промокода для БК Лига Ставок! Этот уникальный код открывает доступ к особым бонусам и усиливает ваш опыт ставок, делая его ещё более выгодным и волнующим. [url=https://ligastavok-promocode.ru/]Промокоды лига ставок[/url] для активации сегодня! С данным промокодом, игроки получают доступ к дополнительным бонусам на ставки, ускоренной процедуре регистрации и эксклюзивным акциям. Воспользуйтесь этой возможностью стать частью элитного клуба пользователей БК Лига Ставок и максимизируйте свои выигрыши с нашим промокодом. Используйте код прямо сейчас и начните выигрывать еще до первой ставки!
Рады сообщить о выпуске эксклюзивного промокода для БК Лига Ставок! Этот уникальный код предоставляет доступ к особым бонусам и усиливает ваш опыт ставок, делая его более прибыльным и волнующим. [url=https://ligastavok-promocode.ru/]Действующие промокоды лига ставок[/url] для активации сегодня! С данным промокодом, игроки получают доступ к бонусным средствам, привилегиям при регистрации и специальным предложениям. Воспользуйтесь этой возможностью стать частью привилегированной группы пользователей БК Лига Ставок и увеличьте свои шансы на успех с нашим промокодом. Активируйте промокод уже сегодня и заберите свои бонусы еще до первой ставки!
На сайте https://animeast.ru/ воспользуйтесь уникальной возможностью посмотреть аниме вместе с друзьями, устроив совместный просмотр. Перед вами огромный выбор вариантов, которые помогут разнообразить досуг и получить от просмотра больше ярких эмоций. Есть как известные аниме, так и новинки, которые понравятся всем любителям этого интересного жанра. Для того чтобы найти интересующий вариант, воспользуйтесь специальным поиском. Изучите каталог аниме, чтобы выбрать оптимальный вариант.
фулфилмент для озон москва https://24fulfilment-marketplace.ru/
Gerchik & Co Group of Companies https://gerchik.co/en is certified through VerifyMyTrade compliance service and provides 5,000 trades on a monthly basis which are then compared to all basic indicators demonstrated by other brokers and liquidity providers.
рулап [url=https://www.rollap.ru]https://www.rollap.ru[/url] .
Приветствуем вас в мир ставок с БК Лига Ставок! Процесс регистрации и входа в личный кабинет созданы для максимального удобства и безопасности каждого пользователя. [url=https://ligastavok-login.ru/]Лига ставок регистрация с бонусом[/url] – легко и быстро, и это открывает доступ к многочисленным возможностям: от ставок на спорт до участия в акциях и получения бонусов.
Начните с нескольких легких шагов для регистрации: заполните обязательные поля формы, подтвердите свою электронную почту и номер телефона, и вы готовы к старту! После регистрации, войдите в личный кабинет с помощью полученных данных – и весь мир ставок БК Лига Ставок окажется у вас под рукой.
Ваш личный кабинет – это командный пункт вашими ставками, где вы можете легко пополнять счет, участвовать в играх, просматривать историю транзакций, а также получать эксклюзивные предложения и уведомления о предстоящих событиях. Не упустите свой шанс – присоединяйтесь к БК Лига Ставок уже сегодня и погрузитесь в мир ставок с комфортом и уверенностью!
Приветствуем вас в мир ставок с БК Лига Ставок! Процесс регистрации и входа в личный кабинет созданы для обеспечения простоты и безопасности каждого пользователя. [url=https://ligastavok-login.ru/]Лига ставок букмекерская регистрация[/url] – легко и быстро, и это открывает доступ к широкому спектру функций: от ставок на спорт до участия в акциях и получения бонусов.
Начните с нескольких легких шагов для регистрации: заполните обязательные поля формы, подтвердите свою электронную почту и номер телефона, и вы готовы к старту! После регистрации, войдите в личный кабинет с помощью полученных данных – и весь мир ставок БК Лига Ставок окажется у вас под рукой.
Ваш личный кабинет – это командный пункт вашими ставками, где вы можете легко пополнять счет, делать ставки, просматривать историю транзакций, а также получать эксклюзивные предложения и уведомления о предстоящих событиях. Не упустите свой шанс – присоединяйтесь к БК Лига Ставок уже сегодня и погрузитесь в мир ставок с комфортом и уверенностью!
Добро пожаловать в мир ставок с БК Лига Ставок! Процесс регистрации и входа в личный кабинет созданы для максимального удобства и безопасности каждого пользователя. [url=https://ligastavok-login.ru/]Лига ставок регистрация с бонусом[/url] – легко и быстро, и это открывает доступ к многочисленным возможностям: от ставок на спорт до участия в акциях и получения бонусов.
Начните с нескольких легких шагов для регистрации: заполните обязательные поля формы, подтвердите свою электронную почту и номер телефона, и вы на пути к успеху! После регистрации, войдите в личный кабинет с помощью полученных данных – и весь мир ставок БК Лига Ставок окажется у вас под рукой.
Ваш личный кабинет – это командный пункт вашими ставками, где вы можете легко управлять своим балансом, делать ставки, просматривать историю транзакций, а также получать эксклюзивные предложения и уведомления о предстоящих событиях. Не теряйте времени – присоединяйтесь к БК Лига Ставок уже сегодня и начните своё путешествие в мире ставок с комфортом и уверенностью!
Добро пожаловать в мир ставок с БК Лига Ставок! Процесс регистрации и входа в личный кабинет созданы для максимального удобства и безопасности каждого пользователя. [url=https://ligastavok-login.ru/]https://ligastavok-login.ru[/url] – легко и быстро, и это открывает доступ к широкому спектру функций: от ставок на спорт до участия в акциях и получения бонусов.
Пройдите несколько простых шагов для регистрации: заполните обязательные поля формы, подтвердите свою электронную почту и номер телефона, и вы готовы к старту! После регистрации, используйте ваш новый аккаунт с помощью полученных данных – и весь мир ставок БК Лига Ставок окажется у вас под рукой.
Ваш личный кабинет – это командный пункт вашими ставками, где вы можете легко пополнять счет, делать ставки, просматривать историю транзакций, а также получать эксклюзивные предложения и уведомления о предстоящих событиях. Не теряйте времени – присоединяйтесь к БК Лига Ставок уже сегодня и начните своё путешествие в мире ставок с комфортом и уверенностью!
Добро пожаловать в мир ставок с БК Лига Ставок! Процесс регистрации и входа в личный кабинет созданы для обеспечения простоты и безопасности каждого пользователя. [url=https://ligastavok-login.ru/]https://ligastavok-login.ru[/url] – легко и быстро, и это открывает доступ к широкому спектру функций: от ставок на спорт до участия в акциях и получения бонусов.
Пройдите несколько простых шагов для регистрации: заполните обязательные поля формы, подтвердите свою электронную почту и номер телефона, и вы на пути к успеху! После регистрации, войдите в личный кабинет с помощью полученных данных – и весь мир ставок БК Лига Ставок окажется у вас под рукой.
Ваш личный кабинет – это центр управления вашими ставками, где вы можете легко управлять своим балансом, делать ставки, просматривать историю транзакций, а также получать эксклюзивные предложения и уведомления о предстоящих событиях. Не упустите свой шанс – присоединяйтесь к БК Лига Ставок уже сегодня и начните своё путешествие в мире ставок с комфортом и уверенностью!
Приветствуем вас в мир ставок с БК Лига Ставок! Процесс регистрации и входа в личный кабинет созданы для максимального удобства и безопасности каждого пользователя. [url=https://ligastavok-login.ru/]Лига ставок вход в личный кабинет[/url] – просто и удобно, и это открывает доступ к многочисленным возможностям: от ставок на спорт до участия в акциях и получения бонусов.
Начните с нескольких легких шагов для регистрации: заполните обязательные поля формы, подтвердите свою электронную почту и номер телефона, и вы готовы к старту! После регистрации, войдите в личный кабинет с помощью полученных данных – и весь мир ставок БК Лига Ставок окажется у вас под рукой.
Ваш личный кабинет – это командный пункт вашими ставками, где вы можете легко управлять своим балансом, участвовать в играх, просматривать историю транзакций, а также получать эксклюзивные предложения и уведомления о предстоящих событиях. Не упустите свой шанс – присоединяйтесь к БК Лига Ставок уже сегодня и начните своё путешествие в мире ставок с комфортом и уверенностью!
Приветствуем вас в мир ставок с БК Лига Ставок! Процесс регистрации и входа в личный кабинет созданы для обеспечения простоты и безопасности каждого пользователя. [url=https://ligastavok-login.ru/]Вход лига ставок[/url] – легко и быстро, и это открывает доступ к многочисленным возможностям: от ставок на спорт до участия в акциях и получения бонусов.
Начните с нескольких легких шагов для регистрации: заполните обязательные поля формы, подтвердите свою электронную почту и номер телефона, и вы готовы к старту! После регистрации, войдите в личный кабинет с помощью полученных данных – и весь мир ставок БК Лига Ставок окажется у вас под рукой.
Ваш личный кабинет – это командный пункт вашими ставками, где вы можете легко пополнять счет, делать ставки, просматривать историю транзакций, а также получать эксклюзивные предложения и уведомления о предстоящих событиях. Не теряйте времени – присоединяйтесь к БК Лига Ставок уже сегодня и начните своё путешествие в мире ставок с комфортом и уверенностью!
Добро пожаловать в мир ставок с БК Лига Ставок! Процесс регистрации и входа в личный кабинет созданы для обеспечения простоты и безопасности каждого пользователя. [url=https://ligastavok-login.ru/]Лига ставок регистрация в конторе[/url] – просто и удобно, и это открывает доступ к многочисленным возможностям: от ставок на спорт до участия в акциях и получения бонусов.
Пройдите несколько простых шагов для регистрации: заполните обязательные поля формы, подтвердите свою электронную почту и номер телефона, и вы готовы к старту! После регистрации, войдите в личный кабинет с помощью полученных данных – и весь мир ставок БК Лига Ставок окажется у вас под рукой.
Ваш личный кабинет – это центр управления вашими ставками, где вы можете легко управлять своим балансом, участвовать в играх, просматривать историю транзакций, а также получать эксклюзивные предложения и уведомления о предстоящих событиях. Не теряйте времени – присоединяйтесь к БК Лига Ставок уже сегодня и погрузитесь в мир ставок с комфортом и уверенностью!
Приветствуем вас в мир ставок с БК Лига Ставок! Процесс регистрации и входа в личный кабинет созданы для максимального удобства и безопасности каждого пользователя. [url=https://ligastavok-login.ru/]Лига ставок мобильная версия вход[/url] – просто и удобно, и это открывает доступ к многочисленным возможностям: от ставок на спорт до участия в акциях и получения бонусов.
Пройдите несколько простых шагов для регистрации: заполните обязательные поля формы, подтвердите свою электронную почту и номер телефона, и вы готовы к старту! После регистрации, войдите в личный кабинет с помощью полученных данных – и весь мир ставок БК Лига Ставок окажется у вас под рукой.
Ваш личный кабинет – это центр управления вашими ставками, где вы можете легко управлять своим балансом, делать ставки, просматривать историю транзакций, а также получать эксклюзивные предложения и уведомления о предстоящих событиях. Не упустите свой шанс – присоединяйтесь к БК Лига Ставок уже сегодня и погрузитесь в мир ставок с комфортом и уверенностью!
CarTrip предлагает широкий выбор комфортабельных автомобилей. Мы поддерживаем автопарк в отличном состоянии и имеем приличный опыт работы. Машину предлагаем заказчику после мойки и чистки. Ищете автодом аренда недорого? Auto-arenda-anapa.ru – сайт, где есть возможность выбрать одну из моделей в доступе. Если будет нужно, мы вам подберем оптимальный вариант. Оплата может быть произведена банковским переводом, наличными или же картами Visa, MasterCard, JCB. С нами вы существенно сэкономите свои денежные средства. Насладитесь прямо из окна автомобиля красотами Анапы!
Организация свадьбы под ключ https://yagodawedding.ru/ с гарантией. Быстрый расчет сметы, работаем с любым бюджетом. Берём на себя абсолютно все свадебные хлопоты. Организуем одни из самых красивых свадебных и семейных событий.
Хотите по недорогой цене снять в СПб жилье? Группа vk.com/komnataspb в этом вам точно поможет. Здесь многие находят себе уютное гнездышко, хотя поиск комнаты и соседей в Санкт-Петербурге – непростое дело. Выбирайте прямо сейчас актуальные предложения. Ищете снять комнату в спб? VK.com/komnataspb – популярное сообщество, в котором больше 6000 человек. Вступите в него и нажмите на рассказать друзьям. Размещение объявлений на стене абсолютно бесплатно, мы тщательно следим за их качеством. Не забудьте ознакомиться с нашими правилами.
Хотите в отменном качестве смотреть онлайн фильмы? Добро пожаловать на Киного! У нас имеется много интересных фильмов, с помощью которых вы после тяжелого дня можете поднять себе настроение. Вы точно забудете о своих проблемах. https://kinogo-fil.net – сайт, специально созданный для людей, любящих кино разных направлений. Теперь не надо идти в кино и тратить деньги на билеты. Смотреть фильмы у нас можно онлайн 24/7 и совершенно бесплатно. Лучшие новинки ждут вас!
SEO раскрутка сайта в топ https://seositejob.ru/ Яндекс и Google от профессионалов.
Приветствуем вас в мир ставок с БК Лига Ставок! Процесс регистрации и входа в личный кабинет созданы для максимального удобства и безопасности каждого пользователя. [url=https://ligastavok-login.ru/]Лига ставок букмекерская контора официальный сайт зарегистрироваться[/url] – легко и быстро, и это открывает доступ к многочисленным возможностям: от ставок на спорт до участия в акциях и получения бонусов.
Начните с нескольких легких шагов для регистрации: заполните обязательные поля формы, подтвердите свою электронную почту и номер телефона, и вы готовы к старту! После регистрации, используйте ваш новый аккаунт с помощью полученных данных – и весь мир ставок БК Лига Ставок окажется у вас под рукой.
Ваш личный кабинет – это центр управления вашими ставками, где вы можете легко пополнять счет, участвовать в играх, просматривать историю транзакций, а также получать эксклюзивные предложения и уведомления о предстоящих событиях. Не упустите свой шанс – присоединяйтесь к БК Лига Ставок уже сегодня и начните своё путешествие в мире ставок с комфортом и уверенностью!
Добро пожаловать в мир ставок с БК Лига Ставок! Процесс регистрации и входа в личный кабинет созданы для максимального удобства и безопасности каждого пользователя. [url=https://ligastavok-login.ru/]Вход в личный кабинет лига ставок[/url] – просто и удобно, и это открывает доступ к многочисленным возможностям: от ставок на спорт до участия в акциях и получения бонусов.
Пройдите несколько простых шагов для регистрации: заполните обязательные поля формы, подтвердите свою электронную почту и номер телефона, и вы готовы к старту! После регистрации, войдите в личный кабинет с помощью полученных данных – и весь мир ставок БК Лига Ставок окажется у вас под рукой.
Ваш личный кабинет – это центр управления вашими ставками, где вы можете легко управлять своим балансом, участвовать в играх, просматривать историю транзакций, а также получать эксклюзивные предложения и уведомления о предстоящих событиях. Не теряйте времени – присоединяйтесь к БК Лига Ставок уже сегодня и погрузитесь в мир ставок с комфортом и уверенностью!
Приветствуем вас в мир ставок с БК Лига Ставок! Процесс регистрации и входа в личный кабинет созданы для обеспечения простоты и безопасности каждого пользователя. [url=https://ligastavok-login.ru/]ligastavok-login.ru[/url] – просто и удобно, и это открывает доступ к широкому спектру функций: от ставок на спорт до участия в акциях и получения бонусов.
Начните с нескольких легких шагов для регистрации: заполните обязательные поля формы, подтвердите свою электронную почту и номер телефона, и вы готовы к старту! После регистрации, войдите в личный кабинет с помощью полученных данных – и весь мир ставок БК Лига Ставок окажется у вас под рукой.
Ваш личный кабинет – это центр управления вашими ставками, где вы можете легко управлять своим балансом, делать ставки, просматривать историю транзакций, а также получать эксклюзивные предложения и уведомления о предстоящих событиях. Не упустите свой шанс – присоединяйтесь к БК Лига Ставок уже сегодня и начните своё путешествие в мире ставок с комфортом и уверенностью!
Приветствуем вас в мир ставок с БК Лига Ставок! Процесс регистрации и входа в личный кабинет созданы для максимального удобства и безопасности каждого пользователя. [url=https://ligastavok-login.ru/]Лига ставок букмекерская регистрация[/url] – легко и быстро, и это открывает доступ к многочисленным возможностям: от ставок на спорт до участия в акциях и получения бонусов.
Начните с нескольких легких шагов для регистрации: заполните обязательные поля формы, подтвердите свою электронную почту и номер телефона, и вы готовы к старту! После регистрации, используйте ваш новый аккаунт с помощью полученных данных – и весь мир ставок БК Лига Ставок окажется у вас под рукой.
Ваш личный кабинет – это командный пункт вашими ставками, где вы можете легко пополнять счет, участвовать в играх, просматривать историю транзакций, а также получать эксклюзивные предложения и уведомления о предстоящих событиях. Не теряйте времени – присоединяйтесь к БК Лига Ставок уже сегодня и погрузитесь в мир ставок с комфортом и уверенностью!
Добро пожаловать в мир ставок с БК Лига Ставок! Процесс регистрации и входа в личный кабинет созданы для обеспечения простоты и безопасности каждого пользователя. [url=https://ligastavok-login.ru/]Лига ставок вход на сайт полная версия[/url] – легко и быстро, и это открывает доступ к широкому спектру функций: от ставок на спорт до участия в акциях и получения бонусов.
Начните с нескольких легких шагов для регистрации: заполните обязательные поля формы, подтвердите свою электронную почту и номер телефона, и вы на пути к успеху! После регистрации, используйте ваш новый аккаунт с помощью полученных данных – и весь мир ставок БК Лига Ставок окажется у вас под рукой.
Ваш личный кабинет – это командный пункт вашими ставками, где вы можете легко пополнять счет, участвовать в играх, просматривать историю транзакций, а также получать эксклюзивные предложения и уведомления о предстоящих событиях. Не упустите свой шанс – присоединяйтесь к БК Лига Ставок уже сегодня и начните своё путешествие в мире ставок с комфортом и уверенностью!
Приветствуем вас в мир ставок с БК Лига Ставок! Процесс регистрации и входа в личный кабинет созданы для обеспечения простоты и безопасности каждого пользователя. [url=https://ligastavok-login.ru/]Бк лига ставок официальный сайт регистрация[/url] – просто и удобно, и это открывает доступ к многочисленным возможностям: от ставок на спорт до участия в акциях и получения бонусов.
Начните с нескольких легких шагов для регистрации: заполните обязательные поля формы, подтвердите свою электронную почту и номер телефона, и вы на пути к успеху! После регистрации, используйте ваш новый аккаунт с помощью полученных данных – и весь мир ставок БК Лига Ставок окажется у вас под рукой.
Ваш личный кабинет – это центр управления вашими ставками, где вы можете легко пополнять счет, участвовать в играх, просматривать историю транзакций, а также получать эксклюзивные предложения и уведомления о предстоящих событиях. Не упустите свой шанс – присоединяйтесь к БК Лига Ставок уже сегодня и погрузитесь в мир ставок с комфортом и уверенностью!
На сайте https://esdogames.ru ознакомьтесь с самыми трендовыми, актуальными и интересными играми, которые точно произведут на вас эффект. Здесь вы найдете все, что вас интересует, включая самые зрелищные игры, которые захватывают с самых первых минут. Также представлен список лучших игр за 2024 год. Вы сможете поиграть во все, что хочется, прямо сейчас. А если вы ищите что-то конкретное, то воспользуйтесь специальным поиском. Подберите игру по жанру, режиму игры, платформе, чтобы облегчить поиск.
нарколог на дом https://someblog.ru/
Доброго!
В мире полно событий, и наша цель – держать вас в курсе всех важных новостей. Мы следим за мировыми тенденциями и изменениями, которые могут повлиять на Украину, и предоставляем вам обзоры и аналитику, чтобы помочь вам понять их значение.
Все самое лучшее на сайте https://smi.in.ua/
[url=https://smi.in.ua/]Последние новости Украины[/url]
новости мира за день
новости Украины видео
новости Украины сегодня
Удачи!
https://giorno24.it/ – sito di informazione per Milano e la Lombardia. Le ultime notizie dalla politica, dall’Italia, dal mondo, dalla cultura, dallo sport e dal calcio, dal settore della moda.
На сайте https://costaspain.net/ вы сможете пообщаться с единомышленниками, узнать ценную, важную информацию на любую тему и получить ответы на самые разные вопросы. На этом форуме можно общаться на самых разных языках, включая русский, украинский, испанский. Здесь смогут оказать помощь, получится просто пообщаться, отдохнуть за приятными разговорами, поговорить об отдыхе, работе, аренде, продаже или покупке недвижимости, ПМЖ в Испании. Можно получить информацию по теме погоды в Испании. Есть возможность ознакомиться с прогнозом погоды. А если интересует что-то конкретное, то воспользуйтесь поиском.
CarTrip предоставляет высококачественные услуги аренды авто в городе Краснодаре по разумной цене. Обращайтесь к нам, если вам необходим прокат авто. Все автомобили находятся в отменном состоянии. Уверенны, что вы станете нашим постоянным клиентом. Ищете авто прокат сервис Краснодар? Prokat.car-trip.ru – портал, где есть возможность узнать условия аренды авто. У нас представлено большое разнообразие машин на любой вкус и кошелек. Мы отлично знаем свое дело и ответим на все ваши вопросы, обращайтесь уже сейчас!
Рады представить выход обновленной мобильного приложения БК Лига Ставок для Android! Этот шаг существенно улучшает ваш пользовательский опыт, делая его более понятным и эффективным. [url=https://ligastavok-android.ru/]Скачать бесплатно лига ставок[/url] сейчас же! В обновлении вы найдете прямой доступ к многообразию спортивных мероприятий из любой точки с помощью вашего устройства. Улучшенное управление профилем, передовая концепция дизайна для удобной навигации и значительно более высокая скорость работы приложения – всё это создано для вас. Присоединяйтесь к числу воодушевленных пользователей и получайте удовольствие от ставок где только захотите и в любой момент. Установите обновление приложения БК Лига Ставок уже сегодня и выходите на новый уровень ставок!
Анонсируем выход новой версии мобильного приложения БК Лига Ставок для Android! Этот шаг полностью преобразует ваш пользовательский опыт, делая его ещё удобнее и эффективным. [url=https://ligastavok-android.ru/]Скачать лига ставок на телефон андроид бесплатно[/url] для всех сейчас! В обновлении вы найдете прямой доступ к огромному ассортименту спортивных мероприятий прямо с вашего смартфона. Усовершенствованное управление профилем, передовая концепция дизайна для простой навигации и значительно более высокая скорость работы приложения – всё это создано для вас. Присоединяйтесь к числу воодушевленных пользователей и наслаждайтесь возможностями от ставок где только захотите и в любое время. Скачайте обновление приложения БК Лига Ставок уже сегодня и переходите к новому этапу игры!
Рады представить запуск новой версии мобильного приложения БК Лига Ставок для Android! Этот шаг существенно улучшает ваш пользовательский опыт, делая его ещё удобнее и эффективным. [url=https://ligastavok-android.ru/]Установить приложение лига ставок[/url] уже сегодня! В обновлении вы найдете прямой доступ к огромному ассортименту спортивных мероприятий из любой точки с помощью вашего устройства. Усовершенствованное управление профилем, передовая концепция дизайна для удобной навигации и значительно более высокая скорость работы приложения – всё это создано для вас. Присоединяйтесь к числу довольных пользователей и получайте удовольствие от ставок в любой точке планеты и в любое время. Скачайте обновление приложения БК Лига Ставок уже сегодня и переходите к новому этапу ставок!
Анонсируем запуск обновленной мобильного приложения БК Лига Ставок для Android! Этот выпуск существенно улучшает ваш пользовательский опыт, делая его более понятным и динамичным. [url=https://ligastavok-android.ru/]Загрузить лига ставок[/url] для всех сейчас! В обновлении вы найдете прямой доступ к огромному ассортименту спортивных мероприятий из любой точки с помощью вашего устройства. Усовершенствованное управление профилем, передовая концепция дизайна для простой навигации и увеличенная скорость работы приложения – всё это создано для вас. Станьте частью довольных пользователей и получайте удовольствие от ставок где только захотите и в любое время. Скачайте обновление приложения БК Лига Ставок уже сегодня и переходите к новому этапу ставок!
Анонсируем запуск новой версии мобильного приложения БК Лига Ставок для Android! Этот шаг полностью преобразует ваш досуг с ставками, делая его ещё удобнее и эффективным. [url=https://ligastavok-android.ru/]Скачать ligastavok[/url] немедленно! В обновлении вы найдете свободу доступа к многообразию спортивных мероприятий прямо с вашего смартфона. Улучшенное управление профилем, инновационная концепция дизайна для удобной навигации и значительно более высокая скорость работы приложения – всё это создано для вас. Присоединяйтесь к числу довольных пользователей и получайте удовольствие от ставок где только захотите и в любое время. Скачайте обновление приложения БК Лига Ставок уже сегодня и выходите на новый уровень ставок!
Лечение лудомании в Алматы https://krasnodar-narkolog.ru/
Центр остеопатии обращаясь, вы получите грамотную помощь в соответствии с высокими стандартами качества. Мы предлагаем для инвалидов и многодетных семей скидки. Контакт – раздел, где вы найдете подробную информацию о том, как к нам попасть. https://osteopati.ru – сайт, где представлены отзывы о центре, ознакомиться с ними можете уже сейчас. Запишитесь на консультацию остеопата. В ходе нее мы выясняем, знакомимся, спрашиваем, какие у вас есть нарушения в организме, объясняем, какой оказывает эффект остеопатическое лечение на эти нарушения.
На сайте https://svoi-rasteniya.ru/ ознакомьтесь со всем ассортиментом питомника. Здесь вы сможете заказать саженцы лимонов, роз, олеандра, инжира, различных декоративных растений. Весь посадочный материал является отборным, качественным, а потому точно приживется даже в суровых российских условиях. Вы сможете осуществить заказ через корзину либо позвонив по телефону на сайте. Регулярно ассортимент расширяется для того, чтобы вы смогли приобрести все, что необходимо и в любом количестве.
На сайте https://moredoram.ru вы найдете дорамы, фильмы, сериалы высокого качества. Они понравятся всем любителям такого жанра и помогут расслабиться после трудового дня и просто получить приятные эмоции. Вы сможете воспользоваться функцией случайной дорамы, изучить список лучших из них. Все они в отличном качестве, с безупречным звуком, что позволит насладиться просмотром на любом устройстве, включая смартфон, ПК, планшет. Регулярно появляются многообещающие новинки, с которыми необходимо ознакомиться и вам.
Вашему вниманию предлагается эксклюзивное предложение от БК Лига Ставок – фрибет для новых игроков! Это бесплатная ставка даёт возможность вам сделать ставку без риска потери собственных средств, открывая широкие возможности для выигрыша с первых же шагов в мире ставок. [url=https://ligastavok-freebet.ru/]Лига ставок вывод фрибета[/url] для всех новичков после регистрации! С фрибетом от БК Лига Ставок, вы имеете возможность испытать все преимущества ставок без каких-либо финансовых обязательств. Это идеальный старт для тех, кто хочет погрузиться в мир ставок с минимальными рисками. Воспользуйтесь этим предложением и начните своё приключение в БК Лига Ставок с нашим фрибетом. Заберите свой фрибет уже сегодня и откройте для себя мир ставок без рисков!
Знакомьтесь с эксклюзивное предложение от БК Лига Ставок – фрибет для новых игроков! Это фрибет даёт возможность вам испытать удачу без риска потери собственных средств, предоставляя широкие возможности для выигрыша с первых же шагов в мире ставок. [url=https://ligastavok-freebet.ru/]Фрибет за регистрацию без депозита лига ставок[/url] для всех новичков после регистрации! С фрибетом от БК Лига Ставок, вы имеете возможность испытать все преимущества ставок без каких-либо финансовых обязательств. Это отличный способ начать для тех, кто хочет погрузиться в мир ставок с минимальными рисками. Воспользуйтесь этим предложением и начните своё приключение в БК Лига Ставок с нашим фрибетом. Заберите свой фрибет уже сегодня и откройте для себя мир ставок без рисков!
Знакомьтесь с эксклюзивное предложение от БК Лига Ставок – фрибет для новых игроков! Это фрибет даёт возможность вам сделать ставку без риска потери собственных средств, предоставляя широкие возможности для выигрыша с первых же шагов в мире ставок. [url=https://ligastavok-freebet.ru/]Лига ставок фрибет за регистрацию без депозита получить[/url] для всех новичков после регистрации! С фрибетом от БК Лига Ставок, вы имеете возможность испытать все преимущества ставок без каких-либо финансовых обязательств. Это отличный способ начать для тех, кто хочет погрузиться в мир ставок с минимальными рисками. Воспользуйтесь этим предложением и начните своё приключение в БК Лига Ставок с нашим фрибетом. Активируйте ваш фрибет сейчас же и стартуйте к выигрышам!
Вашему вниманию предлагается эксклюзивное предложение от БК Лига Ставок – фрибет для новых игроков! Это фрибет позволяет вам испытать удачу без риска потери собственных средств, открывая широкие возможности для получения призов с первых же шагов в мире ставок. [url=https://ligastavok-freebet.ru/]Фрибет за установку приложения лига ставок[/url] новым пользователям после регистрации! С фрибетом от БК Лига Ставок, вы имеете возможность испытать все преимущества ставок без каких-либо финансовых обязательств. Это идеальный старт для тех, кто хочет стать частью мира спортивных ставок с минимальными рисками. Воспользуйтесь этим предложением и начните своё приключение в БК Лига Ставок с нашим фрибетом. Заберите свой фрибет сейчас же и откройте для себя мир ставок без рисков!
Вашему вниманию предлагается эксклюзивное предложение от БК Лига Ставок – фрибет для новых игроков! Это бесплатная ставка даёт возможность вам испытать удачу без риска потери собственных средств, предоставляя широкие возможности для выигрыша с первых же шагов в мире ставок. [url=https://ligastavok-freebet.ru/]Лига ставок фрибет 2222[/url] новым пользователям после регистрации! С фрибетом от БК Лига Ставок, вы имеете возможность испытать все преимущества ставок без каких-либо финансовых обязательств. Это идеальный старт для тех, кто хочет погрузиться в мир ставок с минимальными рисками. Не упускайте свой шанс и сделайте первый шаг к большим выигрышам в БК Лига Ставок с нашим фрибетом. Активируйте ваш фрибет сейчас же и стартуйте к выигрышам!
Вашему вниманию предлагается эксклюзивное предложение от БК Лига Ставок – фрибет для новых игроков! Это бесплатная ставка позволяет вам испытать удачу без риска потери собственных средств, открывая широкие возможности для получения призов с первых же шагов в мире ставок. [url=https://ligastavok-freebet.ru/]Фрибет лига ставок на сегодня бесплатно[/url] новым пользователям после регистрации! С фрибетом от БК Лига Ставок, вы имеете возможность ознакомиться с платформой без каких-либо финансовых обязательств. Это отличный способ начать для тех, кто хочет стать частью мира спортивных ставок с минимальными рисками. Воспользуйтесь этим предложением и начните своё приключение в БК Лига Ставок с нашим фрибетом. Активируйте ваш фрибет уже сегодня и откройте для себя мир ставок без рисков!
Вашему вниманию предлагается эксклюзивное предложение от БК Лига Ставок – фрибет для новых игроков! Это бесплатная ставка даёт возможность вам испытать удачу без риска потери собственных средств, открывая новые горизонты для выигрыша с первых же шагов в мире ставок. [url=https://ligastavok-freebet.ru/]https://ligastavok-freebet.ru/[/url] новым пользователям после регистрации! С фрибетом от БК Лига Ставок, вы получаете шанс испытать все преимущества ставок без каких-либо финансовых обязательств. Это отличный способ начать для тех, кто хочет стать частью мира спортивных ставок с минимальными рисками. Воспользуйтесь этим предложением и сделайте первый шаг к большим выигрышам в БК Лига Ставок с нашим фрибетом. Заберите свой фрибет сейчас же и откройте для себя мир ставок без рисков!
Знакомьтесь с эксклюзивное предложение от БК Лига Ставок – фрибет для новых игроков! Это фрибет позволяет вам испытать удачу без риска потери собственных средств, предоставляя новые горизонты для получения призов с первых же шагов в мире ставок. [url=https://ligastavok-freebet.ru/]Фрибет лига ставок на сегодня бесплатно[/url] для всех новичков после регистрации! С фрибетом от БК Лига Ставок, вы получаете шанс ознакомиться с платформой без каких-либо финансовых обязательств. Это идеальный старт для тех, кто хочет погрузиться в мир ставок с минимальными рисками. Воспользуйтесь этим предложением и начните своё приключение в БК Лига Ставок с нашим фрибетом. Активируйте ваш фрибет уже сегодня и откройте для себя мир ставок без рисков!
Вашему вниманию предлагается эксклюзивное предложение от БК Лига Ставок – фрибет для новых игроков! Это фрибет даёт возможность вам испытать удачу без риска потери собственных средств, открывая новые горизонты для выигрыша с первых же шагов в мире ставок. [url=https://ligastavok-freebet.ru/]Бесплатный фрибет лига ставок[/url] для всех новичков после регистрации! С фрибетом от БК Лига Ставок, вы имеете возможность испытать все преимущества ставок без каких-либо финансовых обязательств. Это идеальный старт для тех, кто хочет стать частью мира спортивных ставок с минимальными рисками. Воспользуйтесь этим предложением и начните своё приключение в БК Лига Ставок с нашим фрибетом. Активируйте ваш фрибет уже сегодня и откройте для себя мир ставок без рисков!
Visit the website https://prm4u.com/ which is the best SMM Panel with automatic fulfillment of your orders. Low price guarantee with high quality services. Take advantage of an effective online tool for social media marketing and search engine optimization.
На сайте https://runobe.ru почитайте ранобэ онлайн и в отличном качестве. Все они на русском языке, а потому насладиться чтением сможет каждый. Все произведения имеют интересный сюжет, непредсказуемую развязку, а потому понравятся всем без исключения. Выбор японских романов обновляется ежедневно, чтобы вы смогли найти то, что действительно вам нравится. Вы можете ознакомиться сразу с несколькими, чтобы определиться с тем, какие из них вам нравятся больше. Для того чтобы подыскать определенный вариант, необходимо воспользоваться специальным поиском.
Как кодируют от алкоголя https://alcoholismcoding.kz/
Появились проблемы психического плана? Артур Евгеньевич Спиглазов – врач-психиатр окажет вам лечение и психологическую помощь. Вы справитесь с тревогами и страхами, почувствуете вкус к жизни и обретете веру в себя. Требуется психиатр для пожилого человека? Jur24.ru – сайт, где можете прямо сейчас записаться на прием к доктору. Артур Евгеньевич – истинный профессионал своего дела. Профессионал подберет подходящие для вас препараты и поставит верный диагноз. С ним всегда можно проконсультироваться при появлении каких-то вопросов, связанных с лечением.
https://vivod-iz-zapoya-dom.ru/
[url=https://myapplestory.ru/]Версия airpods[/url] – это стильный и функциональный аксессуар, который станет вашим незаменимым спутником в повседневной жизни. Ознакомьтесь с нашим ассортиментом реплик и выберите вариант, который подойдет именно вам.
Пользователи ценят долговечность и [url=https://myapplestory.ru/]надежность оригинальных наушников[/url].
На рынке [url=https://myapplestory.ru/]беспроводные наушники apple airpods[/url] зарекомендовали себя как лидеры в своем сегменте.
[url=https://myapplestory.ru/]Рекомендуем обратить внимание[/url] на специальные предложения этого месяца.
Узнайте, где найти [url=https://myapplestory.ru/]доступные AirPods PRO реплики[/url] высокого качества.
Не знаете, где [url=https://myapplestory.ru/]заказать реплику AirPods PRO[/url]? Мы здесь, чтобы помочь.
В нашем магазине вы найдете [url=https://myapplestory.ru/]подлинные реплики AirPods PRO[/url], которые трудно отличить от оригинала.
Рассматривая [url=https://myapplestory.ru/]airpods PRO копия реплика[/url], важно сравнить их функции с оригинальными наушниками.
Мы предлагаем широкий [url=https://myapplestory.ru/]выбор разнообразных реплик беспроводных наушников[/url].
На сайте https://mangaed.ru/ в большом выборе представлены захватывающие, увлекательные комиксы манга, которые вы сможете читать абсолютно бесплатно и в любое время. Все они отличаются непредсказуемым, ярким сюжетом, сопровождаются интересными картинками, которые точно принесут положительные эмоции и помогут вам получить отличное настроение. Все поклонники жанра с нетерпением ожидают новых глав, которые выходят регулярно. Все самые лучшие, интересные и яркие комиксы находятся только на этом сайте, который регулярно радует пользователей.
кредит под развитие малого бизнеса
На рынке есть [url=https://myapplestory.ru/]недорогие аналоги AirPods PRO[/url], удовлетворяющие большинство потребностей.
Ищите [url=https://myapplestory.ru/]доступные альтернативы AirPods PRO[/url]? Мы поможем вам найти идеальный вариант.
[url=https://myapplestory.ru/]Ищете дешевые реплики AirPods PRO[/url]? Не хотите переплачивать за бренд? Ознакомьтесь с нашим ассортиментом и выберите вариант, который подойдет именно вам.
Мы специализируемся на [url=https://myapplestory.ru/]поиск реплик AirPods PRO[/url] для наших клиентов.
[url=https://myapplestory.ru/]Рынке можно встретить[/url] множество интересных предложений.
Мы специализируемся на [url=https://myapplestory.ru/]поиск реплик AirPods PRO[/url] для наших клиентов.
Эта [url=https://myapplestory.ru/]люкс копия[/url] выполнена с исключительным вниманием к деталям.
[url=https://myapplestory.ru/]Беспроводные наушники airpods[/url] идеально подходят для занятий спортом благодаря их легкости и надежности.
Для тех, кто ищет бюджетные [url=https://myapplestory.ru/]airpods PRO копия наушники[/url], существует множество вариантов на рынке.
Русский форум Испании – всё о возможностях для переезда в Испанию из России: https://costaspain.net/viewforum.php?f=66 где обсуждаются бюджетные варианты по учебной визе, визе цифрового кочевника, цены на продукты и проживание, недвижимость, работа в Испании для россиян, обустройство детей в школы, варианты переезда без визы и денег. Также на форуме всегда есть последние новости Испании, красивые фотографии природы и пляжей Испании.
На сайте https://noptur.ru/ подберите экскурсии в Санкт-Петербург, а также другие города страны, которые организуются для школьников, учителей, а также родителей. Перед вами огромный выбор программ, которые подходят как для взрослых, так и детей различного возраста. Туры обойдутся недорого. При этом дети получат море приятных, положительных эмоций. В рамках экскурсионной программы вы увидите самые популярные достопримечательности, а при необходимости ее можно скорректировать. Рассчитайте цену прямо сейчас!
Привет!
Готовьтесь к Черной пятнице на Украине в 2024 году! Это время невероятных скидок и распродаж, которые ждут вас в магазинах и интернете. Узнайте дату проведения и готовьтесь к шопингу с умом!
[url=https://crazysale.marketing/black-friday.html]чорна пятниця[/url]
[url=https://crazysale.marketing/black-friday.html]чёрная пятница[/url]
чорна пятниця 2024
чорна пятниця
чёрная пятница 2024
Удачи!
лечение наркомании цена http://adtherapy.ru/
Эксперты [url=https://myapplestory.ru/]рекомендуем обратить внимание[/url] на эти модели из-за их высокого качества.
В нашем каталоге вы найдете [url=https://myapplestory.ru/]лучшая реплика наушников[/url], которая сравнима с оригиналами.
[url=https://myapplestory.ru/]Рекомендуем обратить внимание[/url] на специальные предложения этого месяца.
Мы нашли для вас [url=https://myapplestory.ru/]недорогие аналоги AirPods PRO[/url], которые стоят внимания.
Не хотите переплачивать за бренд? [url=https://myapplestory.ru/]Версия airpods[/url] – это отличная возможность сэкономить, не жертвуя качеством звука. В нашем магазине вы найдете широкий выбор реплик по доступным ценам.
Если вы хотите сэкономить, попробуйте [url=https://myapplestory.ru/]найти реплику AirPods PRO[/url].
Многие покупатели оценили качество нашей [url=https://myapplestory.ru/]точной копии AirPods PRO[/url].
В этом магазине [url=https://myapplestory.ru/]можно встретить множество[/url] разнообразных аксессуаров.
Приобретение [url=https://myapplestory.ru/]airpods PRO реплика[/url] может быть выгодным решением для экономных покупателей.
На сайте https://turk-kino.ru посмотрите интересное и запоминающееся турецкое кино вместе с другом. Есть такие именитые картины, как «Запах клубники», «Зимородок», «Великолепный век» и многое другое. Для того чтобы начать совместный просмотр, необходимо создать комнату. Сделать это получится в 3 шага, а инструкция прилагается. Перед вами многообещающие новинки, а также топ лучших фильмов за 2024 год. Во всех фильмах играют ваши любимые актеры, звучит приятная музыка. Есть подборка рекомендованных фильмов, которые выбирают многие.
наркозависимый центр https://okcentr.kz/
Привет любители отдыха!
Путешествие — это всегда уникальный опыт, особенно если вы выбираете индивидуальные экскурсии по Калининграду и его окрестностям. Наша команда частных гидов с автомобилями готова погрузить вас в увлекательный мир истории, культуры и природы этого удивительного региона. Одним из главных преимуществ наших экскурсий является индивидуальный подход к каждому клиенту. Мы учитываем ваши интересы, предпочтения и рассчитываем маршрут так, чтобы он максимально соответствовал вашим ожиданиям. Посещение достопримечательностей Калининграда и его окрестностей с нашими профессиональными гидами позволит вам получить максимум удовольствия от путешествия. Наши экскурсии подходят как для индивидуальных туристов, так и для групп различного размера. Ваш комфорт и безопасность для нас превыше всего, поэтому наши автомобили всегда находятся в отличном состоянии. Вы сможете насладиться красотой Калининградской области, не беспокоясь о мелочах. Мы гордимся своими аккредитованными гидами, которые обладают глубокими знаниями и страстью к рассказу о местных достопримечательностях. Узнать больше о наших экскурсиях и забронировать ваше приключение вы можете на нашем сайте: https://topkaliningrad.ru Не упустите возможность погрузиться в удивительный мир Калининграда с командой профессионалов!
экскурсии индивидуальные Калининград
экскурсии обзорные по Калининграду
гид в Калининградской области
экскурсии Калининградская область достопримечательности цены
гиды Калининграда индивидуальные
частные гиды в Калининграде
Удачи и хорошего визита в Калининград!
Оригинальные запасные части и расходные материалы для лабораторного оборудования. https://parts-lab.ru/ – прямые поставки запасных частей и лабораторных приборов в Россию. Работаем напрямую с ведущими производителями: Thermo Fisher Scientific, Agilent, SPECTRO, Dionex. Поставляем колонки газовой хроматографии, капиллярные колонки, расходники для хроматографии, диффузионные насосы, так же запчасти для приборов ионной хроматографии и для элементного анализа. Все для бесперебойной работы вашей лаборатории.
https://propest.ru/kak-vybrat-radiator-dlya-doma.html
Хотите провести незабываемый отпуск в Кисловодске? Тогда забронируйте жилье предварительно. Сделайте это прямо сейчас и наслаждайтесь своим отдыхом. Выбирайте отель, подходящий вам по стоимости и расположению. Ищете Кисловодск мини отели? Kislovodsk-oteli.ru – сайт, где есть вся актуальная информация. Мы подберем для вас лучший вариант. У нас вы узнаете, где лучше забронировать номер. Goldyssun – отель, достойный внимания. Он гарантирует безупречный уровень сервиса. Уверены, что вам он точно подойдет!
На сайте https://klinikaflorovoi.ru/ запишитесь онлайн, чтобы воспользоваться услугами женской консультации. В компании работают компетентные, узкопрофильные специалисты, которые знают, как справиться даже с самым сложным случаем. Ведущие эксперты клиники отличаются огромным стажем, а потому знают, как решить проблему клиентки. Они используют уникальные и новаторские технологии, высокотехнологичное оборудование для того, чтобы правильно выполнить диагностику и назначить правильное лечение. Вам предложат услуги гинеколога, ведение беременности, УЗИ и многое другое.
На этом сайте https://www.rabota-zarabotok.ru/ вы найдете полезную информацию, и отзывы о разных финансовых сайтах. Здесь очень много полезной информации, и разоблачение мошенников. А также узнайте где начать зарабатывать первые деньги в интернете.
Проверка кошельков бумажников на наличие нелегальных средств: Обеспечение безопасности личного электронного активов
В мире цифровых валют становится все более все более необходимо обеспечивать безопасность своих денег. Каждый день обманщики и хакеры создают свежие способы мошенничества и угонов виртуальных средств. Один из ключевых способов обеспечения безопасности является анализ кошелька на выявление подозрительных средств.
Почему именно поэтому важно, чтобы провести проверку собственные электронные кошельки для хранения криптовалюты?
Прежде всего, вот это нужно для того чтобы защиты личных финансовых средств. Большинство участники рынка сталкиваются с риском потери средств своих собственных денег вследствие недоброжелательных планов или угонов. Проверка данных кошелька способствует своевременно выявить сомнительные операции и предупредить.
Что предлагает вашему вниманию фирма-разработчик?
Мы предоставляем услугу проверки цифровых кошельков для хранения криптовалюты и переводов средств с задачей идентификации происхождения средств передвижения и выдачи подробного отчета. Фирма предоставляет система осматривает данные пользователя для идентификации подозрительных операций и определить уровень риска для вашего криптовалютного портфеля. Благодаря нашей службе проверки, вы сможете избежать с регуляторами и обезопасить себя от непреднамеренного участия в незаконных действий.
Как происходит процесс проверки?
Организация наша организация имеет дело с известными аудиторами организациями, например Cure53, для того, чтобы обеспечить гарантированность и адекватность наших проверок. Мы применяем передовые и методы анализа для идентификации опасных действий. Персональные сведения наших граждан обрабатываются и хранятся в специальной базе данных в соответствии с положениями высокими стандартами безопасности и конфиденциальности.
Важный запрос: “проверить свои USDT на чистоту”
Если вас интересует убедиться в надежности собственных USDT кошельков, наши специалисты предлагает возможность бесплатной проверки первых пяти кошельков. Достаточно просто свой кошелек в нужное место на нашем сайте проверки, и мы передадим вам подробный отчет о статусе вашего кошелька.
Обеспечьте безопасность своих финансовые средства в данный момент!
Предотвращайте риски оказаться в жертвой злоумышленников или стать неприятной ситуации нелегальных операций с ваших средствами. Доверьте свои финансы профессиональным консультантам, которые окажут поддержку, вам и вашему бизнесу обезопасить деньги и предотвратить возможные проблемы. Примите первый шаг к обеспечению безопасности защите вашего цифрового портфеля активов уже сегодня!
Компрессоры воздушные https://kompressorpnevmo.ru/ купить в Москве по лучшей цене. Широкий выбор брендов. Доставка по всей РФ. Скидки, подарки, гарантия от магазина.
Витебский госуниверситет университет П. М. Машерова – ведущий образовательный, научный и культурный центр Витебской области. Официальный сайт ВГУ Машерова https://vsu.by новости университета, ВГУ в СМИ, подготовительные курсы Беларусь, выпускники ВГУ Машерова Витебск, международные партнёры. Университет Машерова осуществляет подготовку по предметам: химия, биология, история, физика, программирование, педагогика, психология, математика. Vitebsk State University named P.M. Masherov.
Если хотите смотреть в отменном качестве сериалы и фильмы онлайн, тогда сервис kinogoby.zone/new будет вам точно полезен. Здесь вы найдете все, что вас интересует. Фильмы хорошие, можете смотреть их совершенно бесплатно, не выходя из собственного дома. https://kinogoby.zone/new отличается от других сайтов тем, что предоставляет доступ к большому количеству фильмов. Создатели портала сделали так, чтобы вам интерфейс был понятен. Предлагаем вам расслабиться и обо всем забыть. Выбирайте фильм для просмотра и наслаждайтесь этим процессом!
Привет, поклонники захватывающих приключений! Предлагаю обмениваться своим великолепием от цифрового казино “казино Селектор”. Это не всего лишь сайт для игр, это целый вселенная эмоций и интересов! Здесь я обнаружил все, что мне потребовалось: вариативность игр, щедрые бонусы и скоростные выплаты. Но главное – это настроение, которая правит здесь. Имитированные приключения, удивительные турниры и дружелюбное сообщество игроков делают каждую игру незабываемой! Вступайте к “[url=https://selector-casino-igrat.tech/]Селектор онлайн казино[/url]” и прыгайте в мир интригующих развлечений!
На сайте https://baseus1.ru/ вы сможете приобрести высокотехнологичные, функциональные гаджеты, а также аксессуары к телефонам и многое другое. В каталоге вы найдете кабели для телефонов, автомобильные держатели, зарядные устройства, а также моноподы, защитные стекла, портативные аккумуляторы, микрофоны, колонки. Вся продукция высокого, эталонного качества, а потому точно прослужит долго. Она абсолютно безопасная и надежная. Регулярное пополнение ассортимента. Вся продукция реализуется по доступной стоимости.
Бюро переводов Рокетперевод https://rocketperevod.ru/ в Москве это агентство по переводу текстов и документов. Услуги по профессиональному переводу разных видов документов, таких как с нотариальным заверением, апостилем и консульской легализацией и других. Зайдите на сайт – узнайте стоимость и все преимущества нашей компании.
На сайте https://smsak.org/ вы сможете ознакомиться с топом лучших магазинов аккаунтов. Также есть возможность воспользоваться такой услугой, как платная аренда номеров. Для того чтобы протестировать сервис, доступен бесплатный прием смс. Также вы сможете ознакомиться и с такой информацией, как для чего нужна смс-активация и что представляет собой данная услуга. При этом специально для вас действуют небольшие и привлекательные расценки. Сервис является полностью автоматизированным, а потому услуга будет оказана максимально оперативно.
чистый usdt
Проверка USDT на чистоту: Каким образом обезопасить свои электронные средства
Все больше граждан заботятся в безопасность своих цифровых финансов. Постоянно дельцы разрабатывают новые подходы разграбления цифровых денег, и собственники криптовалюты оказываются жертвами их афер. Один из техник защиты становится проверка кошельков в присутствие противозаконных средств.
С каким намерением это полезно?
В первую очередь, для того чтобы обезопасить личные финансы от шарлатанов и украденных монет. Многие вкладчики встречаются с риском утраты личных финансов из-за хищных планов или хищений. Проверка бумажников помогает обнаружить подозрительные действия а также предотвратить возможные потери.
Что мы предлагаем?
Мы предоставляем сервис проверки цифровых кошельков и операций для обнаружения источника средств. Наша платформа анализирует информацию для определения незаконных действий и также оценки опасности для вашего портфеля. Из-за этой проверке, вы сможете избежать проблем с регулированием и также защитить себя от участия в нелегальных операциях.
Как это действует?
Наша фирма сотрудничаем с ведущими проверочными агентствами, вроде Cure53, с целью предоставить точность наших проверок. Мы используем передовые технологии для обнаружения потенциально опасных сделок. Ваши данные обрабатываются и хранятся согласно с высокими нормами безопасности и конфиденциальности.
Как проверить собственные Tether на нетронутость?
Если вам нужно убедиться, что ваши USDT-кошельки нетронуты, наш сервис предоставляет бесплатное тестирование первых пяти кошельков. Просто введите положение вашего кошелька на на сайте, а также мы предоставим вам полную информацию доклад об его положении.
Защитите вашими активы сегодня же!
Не подвергайте риску подвергнуться обманщиков либо попадать в неприятную ситуацию по причине незаконных операций. Обратитесь за помощью к нашей команде, с тем чтобы сохранить ваши криптовалютные финансовые ресурсы и избежать сложностей. Сделайте первый шаг к сохранности вашего криптовалютного портфеля уже сегодня!
Воздушные компрессоры https://porshkompressor.ru/ в Москве – купить по низким ценам в интернет-магазине. Широкий ассортимент воздушных поршневых компрессоров. В каталоге – передвижные, стационарные модели, с прямым и ременным приводом, сухого сжатия и маслозаполненные.
[url=https://www.grainloader.vn.ua]http://grainloader.vn.ua[/url]
Gold Hobbyist Furnishings stores a trade mark aga of trait texture loaders opportune repayment for jobs of all shapes and sizes.
grain scraper
Готовы к переменам в своей жизни? Купите диплом и начните новую главу!
https://diplom-kuplu.ru
Ищете надежный способ купить автозапчасти для своего автомобиля? Добро пожаловать в интернет-магазин http://autoparts121.ru Мы предлагаем широкий ассортимент оригинальных деталей для различных марок и моделей автомобилей. С нами вы можете быть уверены в качестве запчастей, ведь мы работаем только с проверенными поставщиками. Благодаря удобному сайту с подробными каталогами вы легко найдете необходимые детали и аксессуары. Покупайте с уверенностью в нашем интернет-магазине автозапчастей – ваш автомобиль заслуживает только лучшего!
На сайте https://saffa.ru/ закажите мобильные аксессуары оптом. В разделе вы найдете автомобильные держатели, чехлы, универсальные аксессуары, а также зарядные устройства, портативные аккумуляторы и многое другое. Специально для вас оперативная доставка, которая организуется за 2 дня. Вам доступен безупречный клиентский сервис. Ответы на вопросы вы получите в течение 10 минут. Все товары оригинального качества, имеются гарантии. Всегда доступные расценки за счет сотрудничества напрямую с производителями.
Воздушные компрессоры https://kompressorgaz.ru/ купить по самым низким ценам только у нас с гарантией и бесплатной доставкой. Широкий ассортимент воздушных поршневых компрессоров.
Банкротство физ лиц ДолгХ https://dolgx.ru/ это возможность легально избавиться от долгов. Мы оказываем услуги: составление документов, представление интересов при банкротстве в суде, сопровождение процедуры банкротства. Посетите сайт, узнайте больше и ознакомьтесь со стоимостью услуг.
Хотите настроение себе поднять? Вам поможет Фаномания. Здесь представлено множество смешных мемов, забавных демотиваторов и улетных анекдотов на разные темы. Начните свой день с просмотра позитивных картинок. Ищете стебные отзывы? Funomania.ru – это место, где сможете посмеяться и отвлечься от повседневных забот. Вы узнаете, чем занимался в жизни Мендельсон, какое существо считается зубастым в мире, почему вредно питаться одним Фастфудом. Также здесь есть новая подборка фото сексуальных красоток. Смотрите и получайте удовольствие!
Купить компрессоры https://kompressoroil.ru/ по самым выгодным ценам в Москве в интернет-магазине. Широкий выбор компрессоров. В каталоге можно ознакомиться с ценами, отзывами, фотографиями и подробными характеристиками компрессоров.
Желаете смотреть русские сериалы онлайн в отличном качестве? Тогда добро пожаловать на Ruserial. Мы собрали для вас самый лучший контент. Вы точно останетесь всем, довольны. Предлагаем вам погрузиться в мир интересных историй и эмоций. https://ruserial-full.net/ – портал, который имеет понятный и удобный интерфейс. Здесь есть то, что нужно. Сериалы у вас есть возможность смотреть онлайн абсолютно бесплатно, они вам подарят прекрасные впечатления. Присоединяйтесь к нам уже сейчас и смотрите любимые сериалы.
На сайте https://allgranit.ru/ вы сможете заказать звонок для того, чтобы воспользоваться такой важной услугой, как создание гранитных памятников на заказ. Услуга оказывается не только в Москве, но и по области. Регулярно на определенные товары устанавливаются скидки, акции для того, чтобы ваша покупка была более выгодной. Рассчитать стоимость памятника получится прямо сейчас. Для этого воспользуйтесь специальным калькулятором. Для того чтобы иметь представление о работе, необходимо посмотреть портфолио.
Ищете надежный и удобный способ приобрести необходимые детали для вашего автомобиля? Добро пожаловать в наш интернет-магазин автозапчастей http://partsshop03.ru! В нашем ассортименте вы найдете огромный выбор оригинальных запчастей для различных марок и моделей автомобилей. Мы предлагаем качественные детали по конкурентным ценам. Благодаря удобному сайту с подробными каталогами вы легко найдете нужные запчасти для вашего автомобиля. Покупайте с уверенностью в нашем интернет-магазине — мы знаем все о вашем автомобиле!
We’ll buy or invest in your site and you Investment/Buying ranging from $50,000 to $500,000, depending on stage, market volume, market share sell site
[url=https://www.portcityexteriors.com/about/ ]Tile roofing installation[/url]
[url=https://chat-hozn3.com/vb/showthread.php?p=1823673#post1823673]A Comprehensive Overview of Roof Flashing[/url] 0d05900
Киного https://kinogo.vision/ это возможность смотреть фильмы, сериалы, мультфильмы онлайн бесплатно в высоком качестве. Все новинки 2024 уже на сайте. Коллекции за предыдущие годы. ТОП фильмов, сериалов где каждый найдет для себя необходимый жанр.
Discovered an article that will surely interest you – I recommend checking it out https://tonirovkaforum.bestff.ru/viewtopic.php?id=3737#p17253
Продвижение сайтов в поисковых системах https://seoshnikiguru.ru/ с гарантией результата. SEO продвижение сайтов в ТОП-10 Яндекс, заказать поисковое сео продвижение, раскрутка веб сайта в Москве.
LastTorrent.ru предлагает вам через торрент скачать понравившуюся игру. Вам не придется долго ждать. У нас есть удобный рубрикатор, с помощью которого вы найдете то, что вам нужно. Мы не загромождаем страницы ненужными баннерами. Не обязательно для доступа к файлам торрентов проходить регистрацию. https://lasttorent.ru – популярный сайт, вы получите полноценный продукт, без риска заражения своего компьютера вирусами, если загрузите игры здесь. У вас появились вопросы? Тогда обращайтесь к нам, мы всегда готовы вам помочь.
Productive Backlinks in Weblogs and Remarks: Boost Your SEO
Links are essential for enhancing search engine rankings and raising site visibility. By incorporating hyperlinks into blogs and comments smartly, they can considerably boost visitors and SEO efficiency.
Adhering to Search Engine Algorithms
The current day’s backlink positioning tactics are meticulously tuned to line up with search engine algorithms, which now emphasize website link high quality and relevance. This guarantees that hyperlinks are not just numerous but meaningful, guiding end users to beneficial and relevant content material. Website owners should concentrate on integrating backlinks that are situationally suitable and boost the general content material good quality.
Benefits of Utilizing Fresh Donor Bases
Utilizing up-to-date contributor bases for hyperlinks, like those managed by Alex, offers significant rewards. These bases are regularly refreshed and consist of unmoderated sites that don’t attract complaints, making sure the links put are both powerful and certified. This method assists in sustaining the usefulness of backlinks without the pitfalls linked with moderated or troublesome resources.
Only Approved Resources
All donor sites used are approved, steering clear of legal pitfalls and adhering to digital marketing standards. This determination to making use of only approved resources assures that each backlink is legitimate and trustworthy, thereby developing trustworthiness and reliability in your digital presence.
SEO Impact
Skillfully placed backlinks in blogs and remarks provide more than just SEO advantages—they boost user experience by connecting to relevant and top quality articles. This technique not only satisfies search engine criteria but also engages users, leading to better targeted traffic and improved online proposal.
In essence, the right backlink technique, specifically one that utilizes refreshing and trustworthy donor bases like Alex’s, can transform your SEO efforts. By focusing on high quality over quantity and adhering to the latest requirements, you can make sure your backlinks are both powerful and effective.
Покупайте необходимые детали для вашего автомобиля в интернет-магазине автозапчастей http://shopauto74.ru! Вас ждет широкий выбор оригинальных запчастей для автомобилей различных марок и моделей. Мы предлагаем огромный выбор качественных запчастей. В нашем ассортименте вы найдете все, что нужно вашему автомобилю: от аксессуаров до самых важных деталей. Воспользуйтесь удобным каталогом оригинальных запчастей и вы найдете все что нужно. Вы приятно удивитесь низким ценам и широкому выбору. Доверьте свой автомобиль нашему интернет-магазину, и он будет всегда в надежных руках!
On the website https://magicday.ae/ you can order live, exciting and interesting performances by actors, dancers, animators. You will find enchanting, luxurious costumes, a great and friendly atmosphere, as well as artists who will definitely not leave anyone indifferent. And most importantly, it will be possible to invite artists to absolutely any holiday. Check out the portfolio right now to find out how the performances are going. The artists will arrive at the agreed time. All services are provided at attractive prices.
Заказать SEO продвижение сайтов https://seoshnikigo.ru/ в ТОП поисковых систем Яндекс и Google в Москве, оплата за результат и по факту. Кейсы, стратегии продвижения, скидки и акции, индивидуальный подход
Компания «MIRTASH» за время деятельности уже приобрела постоянных заказчиков, она зарекомендовала себя как достойный и надежный партнер. Предлагаем качественный камень и отличный сервис. В нашем распоряжении свое производство, у нас всегда имеются складские запасы. https://mirtash.ru/ – сайт, где вы можете оставить свои контактные данные. Мы в самое ближайшее время с вами свяжемся. Если вам интересны изделия из доломита, лемезита и других пород камня, обращайтесь смело к нам, мы быстро доставляем продукцию до объекта по всей РФ.
Хотите получить позитивные эмоции? Тогда добро пожаловать на ЗАСОС.ФАН! Это развлекательный портал, который поднимет ваше настроение. Мы заставим вас посмеяться от души. Ищете барышни? Zasos.fun – сайт, где представлен отборный юмор и веселые мемы. Здесь найдете качественные фотографии очаровательных девушек в сексуальных нарядах, удивительные снимки с животными и птицами. Также узнаете, какой самый очевидный намек от представительницы прекрасного пола вы упустили. У нас есть множество прикольных картинок, заходите к нам в гости!
Купить квартиру в Казани https://novostroyzhilie.ru/ от застройщика. Планировки и цены трехкомнатных, двухкомнатных и однокомнатных квартир в новостройке.
На сайте https://dolgovnet82.ru вы сможете уточнить всю важную информацию, которая касается такой важной услуги, как банкротство физических лиц. Вас избавят от долгов, а также кредитов законным путем. За счет того, что обращение происходит к арбитражному управляющему напрямую, то вы сможете сэкономить приличную сумму денег. Процедура будет начата в день обращения, и при этом не нужно будет вносить платежи. Ваше имущество будет сохранено, как и покой близких. В этой компании действует привлекательная стоимость.
Opened up interesting material – I recommend sharing this discovery http://igenplan.ru/forum/user/92861/
Хотите смотреть интересные фильмы и сериалы совершенно бесплатно и в отличном качестве? Лордс вам в этом поможет. Вы можете, посмотреть любимый фильм онлайн и не ждать пока его покажут по телевизору. Проведите свое свободное время радостно. smotret.lords.lat – https://smotret.lords.lat здесь вы всегда сможете удобно смотреть фильмы онлайн. Безупречное качество, огромная база, ненавязчивый интерфейс сделают незабываемым домашний киносеанс. Фильмы, которые мы предлагаем на нашем портале, избавят вас от депрессии, вы можете их уже сейчас начать смотреть!
На сайте https://dokumentooborot24.ru/ ознакомьтесь с инновационной и уникальной программой «1С:Документооборот». Такая версия была создана специально для тех предприятий, у которых имеются дочерние компании, а также филиалы. Дополнительно ей могут пользоваться и те, у кого бизнес за рубежом, но развивают его на территории России. Функциональная программа помогает автоматизировать все процессы, включая проработку черновиков, а также уничтожение документации. Кроме того, программа дает возможность улучшить качество обслуживания среди сотрудников, оптимизировать их труд.
On the website https://expertoption-app.online/ you can view and download the Expert Option application for all devices and you will have instant access to trading anywhere. Expert Option is a high level options trading with over 150 assets to trade. More details on the website.
На сайте https://vernissazh.ru забронируйте гостевой дом «Вернисаж». До пляжа, набережной, моря всего несколько метров. А с террасы каждый гость получает возможность насладиться живописным, красивым видом. Почти все развлечения, а также достопримечательности, аттракционы расположены в нескольких метрах. В гостевом доме находятся 14 номеров. Круглосуточное размещение гостей, а на территории находится бесплатный интернет. При необходимости будет организована встреча из Симферополя. Гости смогут воспользоваться бесплатной парковкой.
Написание курсовых работ https://courseworkskill.ru/ на заказ быстро, качественно, недорого. Сколько стоит заказать курсовую работу. Поручите написание курсовой работы профессионалам.
Вам необходимы качественные услуги электрика, гарантии по доступной стоимости и в сжатые сроки? Тогда непременно звоните нам. Вы получите профессиональную консультацию. Квалифицированные сотрудники используют исключительно современное оборудование, они готовы выполнить электромонтажные работы любой сложности. Легко им доверьтесь. Ищете электромонтажные работы по разумным ценам? Electrician-perm.ru – сайт, где представлены услуги и цены. Мы ответственно подходим к заказам и удовлетворим ваши потребности. Предлагаем способы оплаты удобные. Работаем исключительно для вас, обращайтесь!
The spa 4hands invites find out one of the varieties massage techniques, is what we do. What is an masseuses interested in everyone. massage with stones is the art of giving for pleasure. You be surprised to that,what variety enjoyment can get to know from choice massage. In studio Workshop relaxing massage masseurs can do good medical massage.
How is it done, and is there something exotic? We will tell you all about him that you wanted to know |Our swedish massage is visited not only by men but also by women, and also by couples. You want to enjoy is exactly what infinitely … Our а task this is to please personally you awesome in many ways adult massage. Distinctive approach to all yours requirements and claims.
The beautiful masseuses our the spa salon will give you an unforgettable experience. The spa salon is a place of rest and relaxation. Such adult massage, as in principle, and relaxation, affects on some area naked body, this allows male gain strength. Your best stop choice not on one masseuse, choose two girls! Choose for yourself masseur girl by external data, both professional and professional skills!
Spa center in Empire State provide luxurious rooms with convenient interiordecoration. All of these accommodation promote to stay with you incognito.
We have a showroom in Manhattan. Women Amelia :
[url=https://nude.manhattan-massage.com]nude massage sexy[/url]
На сайте https://ar26.ru/ специально для вас подберут наиболее выгодную, подходящую под все параметры недвижимость, которая будет доступна по цене. Кроме того, специалисты подготовят все необходимые документы, чтобы сделка прошла наиболее комфортно для вас. Сотрудники не только помогут найти лучший вариант, но и оформят сделку, гарантируют отличный результат. Также на сайте вы отыщите полезные, информативные новости на данную тему. Все специалисты отличаются высокой квалификацией, чтобы предоставить услуги на должном уровне.
Велнес-клуб премиум-класса BIOSFERA https://biosfera-club.ru/ на Ленинском проспекте и Шаболовской это тренажерный зал, фитнес и групповые программы, бассейн с банным комплексом, SPA-салон и инновационный центр функциональной диагностики. Зайдите на сайт – узнайте больше об услугах и их стоимости.
Vavada casino telegram https://vavada-telegram.com/
На сайте https://newturkishserials.ru/ ознакомьтесь с топом самых популярных турецких сериалов, которые понравятся всем, кто любит интересный, незаурядный сюжет, яркие, положительные эмоции. На странице вы получите полную, исчерпывающую информацию о таких сериалах, как: «Великолепный век», «Бесконечная любовь», «Любовь напрокат», «Судьба», «Сыла: Возвращение домой». Вы узнаете о каждом сериале, сюжете, а также моральных, эмоциональных моментах. Кроме того, получите информацию о том, насколько популярны турецкие сериалы и почему они нравятся многим.
На сайте https://incubatoreggs.ru/ закажите качественное, экологически чистое инкубационное яйцо индейки, курицы, утки, гуся, а также различных других домашних птиц. Вся продукция поставляется от ведущих фабрик России, Европы. Компания работает без выходных, а бесплатная доставка от 3000 рублей. У компании имеются пункты выдачи, чтобы вы смогли забрать продукцию в целости. А если хотите найти что-то определенное, то воспользуйтесь специальным поиском. Все яйцо всегда свежее, ароматное и подходит для приготовления различных блюд.
На сайте https://pravovoivihod.ru пройдите тест для того, чтобы узнать, подходит ли вам бесплатная процедура, предполагающая списание долга через МФЦ. При этом вы сможете получить еще и 2 подарка. Вы сможете списать долги, кредиты, сумма которых не превышает 200 000. У компании огромный опыт работы – 8 лет в области банкротства. Именно поэтому с вашей проблемой, независимо от ее сложности, справятся в минимальные сроки. Более 1 500 человек уже успешно списали долги, почти 2,6 млрд. кредитов отдали.
Came across a unique article – it’s worth your attention https://1001viktorina.ru/forum/viewtopic.php?f=19&t=625&sid=403c3f073b41ba49ea6bf4aca96cfb5f
На сайте https://pro100-stone.ru рассчитайте то, сколько будет стоить изготовление, производство изделий, выполненных из искусственного камня. В этой компании получится заказать подоконники, столешницы, раковины, мойки, ресепшен и многое другое. Есть возможность создать как горизонтальную, так и вертикальную поверхность. Это отличный вариант для самых разных помещений, включая рестораны, учебные заведения, офисы, магазины. Все изделия отличаются высокой практичностью, надежностью.
link building
Backlink creation is just as efficient now, simply the resources to operate in this area possess changed.
You can find numerous possibilities for backlinks, our company employ a few of them, and these approaches operate and have already been examined by our experts and our customers.
Not long ago we conducted an test and it transpired that less frequent searches from just one domain rank nicely in online searches, and this does not need to be your own website, it is possible to utilize social media from the web 2.0 range for this.
It additionally possible to in part shift mass through web page redirects, offering a diverse link profile.
Visit to our web page where our company’s solutions are actually presented with thorough descriptions.
Почему посудомоечная машина https://kulbar.ru/2024/01/21/pochemu-posudomoechnaya-mashina-eto-neobhodimost-dlya-sovremennogo-doma/ необходимость для современного дома? Как использовать и как выбрать посудомойку?
Купить комплект тюнинга https://autoupgrade.by/
Creating distinct articles on Medium and Telegraph, why it is essential:
Created article on these resources is superior ranked on less frequent queries, which is very vital to get natural traffic.
We get:
organic traffic from search algorithms.
organic traffic from the in-house rendition of the medium.
The platform to which the article refers gets a link that is valuable and increases the ranking of the webpage to which the article refers.
Articles can be made in any quantity and choose all less common queries on your topic.
Medium pages are indexed by search algorithms very well.
Telegraph pages need to be indexed separately indexer and at the same time after indexing they sometimes occupy spots higher in the search engines than the medium, these two platforms are very valuable for getting traffic.
Here is a URL to our services where we offer creation, indexing of sites, articles, pages and more.
На сайте https://detskoe.info/ подберите промокоды, есть информация о распродажах, скидках, а также акциях. Все это позволит приобрести детские вещи, мебель и все необходимое по привлекательной стоимости. Здесь вы найдете и такие товары, за покупку которых предоставляется подарок. На распродажах вы сможете приобрести сразу несколько вещей в гардероб. Для того чтобы приобрести все, что нужно, необходимо просто изучить все необходимые предложения в магазинах, после чего выбрать самые подходящие. Все вещи качественные и прослужат долго.
На сайте https://ruserial-full.net/ посмотрите все самые интересные, увлекательные сериалы за прошлые или нынешние года. Для того чтобы сориентироваться в выборе, необходимо определиться с жанром. В каталоге находятся драмы, боевики, триллеры, мистика, а также детективы, криминал, комедии, мелодрамы. Все это в отличном качестве, с хорошим звуком, поэтому доставит только положительные эмоции. Изучите раздел с самыми популярными фильмами, которые просматривает большинство. Здесь также находятся и новинки.
Продажа квартир в Казани https://kupitkvartiruzdes.ru/ от застройщика. Большой выбор квартир. Возможность купить онлайн. Квартиры с дизайнерской отделкой.
На сайте https://pluryal.info/ каждый желающий получает возможность заказать филлеры, которые отличаются премиальным качеством из Европы. Продукция представлена более чем в 70 странах. Компания находится на рынке более 13 лет, а потому заполучила признание постоянных клиентов. Препараты имеют сертификаты качества, что подтверждает серьезные намерения. Имеются филлеры для повышения упругости, сияния кожи, придания свежего вида и бархатистости, а также те, что корректируют морщины, гусиные лапки.
Car-scales.ru предлагает вам купить по доступной цене автомобильные весы. Мы ценим и любим своих клиентов. Гарантируем индивидуальный подход. Обращаясь к нам, вы получите исключительно высококачественную продукцию. У нас имеется то, что вам нужно. Ищете автомобильные весы купить москва? Car-scales.ru – сайт, где можете в любое удобное для вас время заказать автовесы. Поставка будет осуществлена точно в указанный срок. Мы уделяем особенное внимание уровню обслуживания. С удовольствием поможем вам сделать оптимальный выбор и ответим на все вопросы. Звоните нам прямо сейчас!
курс эксель онлайн – Обучение с гарантиями государственного университета.
На сайте https://oknaksa.ru/ оформите заявку для того, чтобы вызвать замерщика на дом. Специально для вас доступны любые типы остекления для квартир, домов. И все в одном месте. На все работы предоставляются гарантии до 20 лет. Доступно бесплатное обслуживание, работы проводятся по ГОСТу, в соответствии с нормативами. На объект приезжают сертифицированные сотрудники, опыт которых более 5 лет. Они предложат различные варианты решения вопроса. Компания заполучила огромное количество постоянных и довольных клиентов, которые рекомендуют ее своим друзьям.
На сайте https://nashi-teplicy.ru/ напишите сообщение для того, чтобы заказать производство теплиц из поликарбоната как в Москве, так и области. Разработка происходит по индивидуальным эскизам заказчика. В каталоге представлены как прямостенные, так и арочные, каплевидные теплицы, парники, навесы. Все они самых разных размеров, а потому сможете подобрать решение под свои предпочтения, требования. На продукцию установлены привлекательные расценки. Также есть и спецпредложения. Организуется доставка по всей России, СНГ.
Привет всем!
С нами вы всегда будете в курсе всех важных событий в Украине и за рубежом. Мы предоставляем вам полную и достоверную информацию
Все самое лучшее на сайте https://10minut.info/category/sport/
[url=https://10minut.info/category/ecomomika/]Новости Экономики[/url]
дтп видео
Новости Украины лента
авто новости
Удачи!
Mostbet Casino регистрация telegram https://mostbet-telegram.com/
«ЛУКС» была создана как строительная компания Крыма в 2015 году.
Компания предлагает строительство домов в Крыму и Севастополе,
на сайте компании уже более 100 готовых проектов для Крыма.
лукс крым
https://evaplan-spb.ru/2024/05/03/Решаем-задачи-по-физике-онлайн-помощники
аккумулятор автомобильный купить в москве
Купить квартиру в новостройке https://newhomesale.ru/ в Казани. Продажа новой недвижимости в ЖК новостройках по ценам от застройщика.
В поисках незабываемого отдыха на Байкале? Ищете надежное турагентство, которое поможет вам спланировать вашу поездку? FanatBaikala.ru – идеальный выбор для вас! Здесь вы найдете самые увлекательные предложения от [url=https://fanatbaikala.ru/tours]турагентства Иркутска на Байкал[/url] и [url=https://fanatbaikala.ru/tours]турагентства Байкал[/url].
FanatBaikala.ru предлагает широкий спектр маршрутов, которые удовлетворят даже самые взыскательные вкусы. От увлекательных экскурсий до расслабленного отдыха на берегу озера – здесь каждый найдет что-то по душе.
Не упустите возможность сделать ваш отдых незабываемым с FanatBaikala.ru!
Стальные трубчатые радиаторы Arbonia (Чехия) и Rifar Tubog (Россия) https://medcom.ru/forum/user/226934/ подходят как для частных домов, так и для квартир в многоэтажках.
brows and lips
На сайте https://sobr26.ru получите бесплатную консультацию для того, чтобы узнать все про такую важную и необходимую услугу, как охрана недвижимости, важного объекта. Компания охраняет различные объекты, гарантирует безопасность имущества. Она работает в данном направлении более 20 лет, а потому отличается колоссальным опытом. Все услуги предоставляются «под ключ». Здесь получится заказать, в том числе, и пультовую, личную охрану, а также охрану массовых предприятий. Охрана объекта различного уровня сложности.
фен дайсон суперсоник [url=https://dyson-feny.com/]https://dyson-feny.com/[/url] .
Читайте больше о подборе машины https://blamper.ru/auto/zachem-nuzhen-agregator-dlya-poiska-avtomobiley-po-vsey-rossii.html
трубчатые радиаторы подходят как для частных домов, так и для квартир в многоэтажках.
Вы интересуетесь магией? Заходите на сайт мага Азала. Здесь точно найдете для себя что-то новое. Узнаете, как действует приворот, и какие виды бывают, есть ли домовой в доме, что такое спиритизм и другое. Вы можете заказать магическую консультацию уже сейчас. Ищете активная защита? Magomagii.ru – сайт, где можно найти стоимость помощи мага. Здесь представлены статьи о магии, где Азал вложил все свои умения, опыт, знания и душу. Вопросы в комментариях получают свои ответы. Маг будет рад вас видеть на своем ресурсе.
На сайте https://taker12.casino поиграйте в интересные игры, которые понравятся своей динамикой, яркостью, а также интересным подходом. Вы сможете заработать себе на мечту, а выигранные средства вы получите очень быстро, а вывести их можно наиболее удобным способом. Прямо сейчас попытайте удачу в самых популярных слотах, которых здесь огромное количество. Не упустите возможность сыграть в Live-игры. Для всех клиентов действуют наиболее выгодные условия, привилегии, бонусы. Вы сможете заработать до 15% по реферальной программе.
Came across an intriguing article – it’s worth your attention, trust me http://nsk.ukrbb.net/viewtopic.php?f=41&t=26370
I have been dreaming about lately. The specifics on the network is excellent and needed and is going to help my friends at work in our studies all the time. It shows that everyone here acquired a lot of details about interesting topics and other subjects and info like wise show it. I’m not typically on the net during the week but when I am bored I am more often than not looking for this kind of knowledge and things closely having to do with it. If anyone gets a chance, take a look at my website. [url=https://bioscienceadvising.com/how-write-grant-part-3-main-body-abstract-preparation]methods to uphold privacy in grant composition procedures[/url]
На сайте https://www.youtube.com/@mozspb вы сможете ознакомиться с информацией, а также видеоматериалами от популярного геймера Алексея, который проживает в Санкт-Петербурге. Ему 41 год и он особенно любит играть в шутеры. А после знакомства с Михаилом Алексей плотно подсел на такую игру, как PUBG. А в феврале 2024 его уговорили на то, чтобы он начал стримить. Алексей будет рад всем, кто хочет присоединиться и погрузиться в мир игр. Всего на канале более тысячи подписчиков, размещено 29 видео. Канал был зарегистрирован 16 ноября 2019 года.
Продажа квартир в новостройках https://newflatsalespb.ru/ СПБ по выгодным ценам от застройщика. Купить квартиру в СПБ на выгодных условиях.
свадебный салон Санкт-Петербург, где более 400 платьев в наличии. Свадебные и вечерние платья А-силуэта, прямые, греческие, пышные, силуэт Рыбка.
На сайте https://premiumface.ru/ почитайте информацию об уникальной и инновационной процедуре многоуровневой комплексной подтяжке лица. Для того чтобы получить необходимый результат, идеальное лицо, используются исключительно высокотехнологичные Европейские препараты премиального уровня. Они созданы на базе гиалуроновой кислоты, различных полезных добавок, которые восстанавливают кожу, придают ей упругость, эластичность, роскошный внешний вид. Такая процедура обязательно вам подойдет, если желаете выглядеть ухоженно, но без одутловатого лица, стремитесь сохранить естественную красоту.
https://service.gadgetufa.ru/remont-smartfonov/remont-avtomagnitol/
Все для рукоделия, декор, вышивания и украшения. Наборы для канцелярии, росписи текстиля, декорации и детского творчества.
Stumbled upon a unique article, I suggest you take a look http://almet.listbb.ru/ucp.php?mode=login&sid=dbe7cd6158c6e4e03f8555d688b8fc68
На сайте https://antonenko-media.com оставьте заявку для того, чтобы заказать видеосъемку не только в городе, но и по области. Перед вами весь перечень услуг как по фотосъемке, так и видеосъемке. Компетентные, проверенные сотрудники без труда и на профессиональном уровне организуют онлайн-трансляцию любого мероприятия. И самое главное, что все пройдет именно так, как и нужно. Работы выполняются только на инновационном, высокотехнологичном оборудовании. Работать со специалистами компании всегда приятно, непринужденно.
Компания «Growex Group» более 15 лет оказывает полный комплекс услуг в международном логистическом направлении. Основное направление деятельности заключается в таможенном оформлении, а также контейнерных перевозках, подробнее об оказываемых услугах Вы можете ознакомиться на https://growex-group.ru/ Закажите перевозки автомобильным, морским, воздушным, железнодорожным транспортом.
цена дайсон фен [url=https://www.dyson-feny.com]https://www.dyson-feny.com[/url] .
На сайте http://kinomanhd.vip вы найдете огромное количество сериалов, фильмов в отличном качестве. Есть новые сериалы, фильмы за 2023 и 2024 года. Смотрите все, что хотите в любое время и без регистрации. Есть возможность подобрать оптимальный вариант по актерскому составу, году производства, жанру, режиссерам, стране производства. Имеются подборки про врачей, девушек, маньяков, любовь. Просматривайте фильмы в пятничный вечер или после работы. Картинка эталонного качества, хороший звук.
https://nz-offers.pages.dev/
反向链接金字塔
G搜尋引擎在多次更新後需要使用不同的排名參數。
今天有一種方法可以使用反向連接吸引G搜尋引擎對您的網站的注意。
反向連接不僅是有效的推廣工具,也是有機流量。
我們會得到什麼結果:
我們透過反向链接向G搜尋引擎展示我們的網站。
他們收到了到該網站的自然過渡,這也是向G搜尋引擎發出的信號,表明該資源正在被人們使用。
我們如何向G搜尋引擎表明該網站具有流動性:
個帶有主要訊息的主頁反向鏈接
我們透過來自受信任網站的重新定向來建立反向連接。
此外,我們將網站放置在独立的網路分析器上,網站最終會進入這些分析器的高速缓存中,然後我們使用產生的連結作為部落格、論壇和評論的重新定向。 這個重要的操作向G搜尋引擎顯示了網站地圖,因為網站分析器顯示了有關網站的所有資訊以及所有關鍵字和標題,這很棒
有關我們服務的所有資訊都在網站上!
На сайте http://nemans.ru вы сможете приобрести всю необходимую продукцию, включая пакеты с ручками, фасовочные пакеты, мешки полипропиленовые, пленку армированную, полиэтиленовую. Также в разделе имеется ветошь, ароматное мыло, перчатки, черенки, бытовая химия, ведра и многое другое, что вы сможете заказать прямо сейчас. Среди рекомендованных товаров находятся вязаные перчатки, туалетная бумага, хозяйственное мыло, неткол. Вся продукция отличного качества, надежная и наделена длительными сроками эксплуатации.
Сайт https://центрпрофмонтаж.рф/ это установка септиков из бетонных колец в Московской области, а также автономных станций ЛОС для частных домов и дач.
https://gamesdb.ru/
фен стайлер дайсон [url=http://www.dyson-feny.com/]http://www.dyson-feny.com/[/url] .
дайсон фен с насадками [url=http://www.dyson-feny.com]http://www.dyson-feny.com[/url] .
дайсон купить фен [url=https://www.dyson-feny.com]https://www.dyson-feny.com[/url] .
Мастер Азарных Илья Владимирович имеет нужные навыки, умения и знания. В своей профессиональной деятельности он не останавливается на достигнутом результате. Клиенты остаются довольными от сотрудничества с ним, так как он гарантирует высочайшее качество предлагаемых услуг, оперативность, а также лояльные цены. Ищете ремонт лазерного принтера самсунг недорого? Zipprinters.ru – сайт, где представлена контактная информация специалиста. Обращайтесь к истинному профессионалу. Илья Владимирович поможет решить вашу проблему и будет рад вас видеть среди своих клиентов!
https://novyidomkupitspb.ru/ купить квартиру в новостройке Санкт-Петербурга от застройщика
На сайте https://sobr26.ru ознакомьтесь с номером телефона охранной организации СОБР. Здесь оказываются услуги, связанные с охраной недвижимости, организуется безопасность объекта, есть возможность воспользоваться физической охраной, которая оказывается более 20 лет. Прямо сейчас вы сможете рассчитывать на бесплатную консультацию. Каждый клиент сможет организовать пультовую, физическую охрану, пожарную сигнализацию, видеонаблюдение. Для того чтобы воспользоваться услугами, оставьте заявку непосредственно на сайте.
Онлайн-заведение «Vavada» предлагает сыграть в увлекательные и интересные игры, а заодно и заработать на своем увлечении. На сайте представлен лицензированный контент, что дает возможность выиграть крупную сумму. Казино было основано в 2017 году, и за это время стало одним из самых популярных. Работает по лицензии Кюрасао. На сайте https://vavadaplay3.com/ ознакомьтесь с условиями игры и возможностями.
Мобильная версия бк Зенит zenitbet1.com
По запросу [url=https://zenitbet1.com/]zenit win[/url] вы на верном пути. Зеркало официального сайта Zenitbet легко осуществляет работу на территории России и в полном объеме надежно. Вы можете без опасений давать свои данные и быть уверены в том, что данные не могут быть заняты другими пользователями. Также средства на вашем счету окажутся под защитой. Комфорт в том, что сайт соответствует официальному и Вам не нужно будет привыкать к новой картинке. А еще не нужно проходить вторичную регистрацию, если вы уже были зарегистрированы. В зеркале сохраняются Ваши пароли, впишите их и заходите в свой кошелек.
https://newflatstroyka.ru/ квартиры от застройщика в Казани
Сайт https://steel-plant.ru/ это производство и продажа кровельно-фасадных материалов: водосточных систем, доборных элементов, а также стальных оцинкованных листов с цветным полимерным покрытием по RAL.
На сайте http://ttps//lovely-professional.ru вы сможете приобрести качественные материалы, которые используются для наращивания ресниц. При этом установлены привлекательные расценки на всю продукцию. Она представлена именитыми брендами, которые отвечают за качество. Товары поставляются через официальных дистрибьюторов. В разделе представлены ресницы, товары для биозавивки, ламинирования, пинцеты. Есть возможность заказать продукцию оптом. Действуют привлекательные расценки. Продукция сертифицированная.
dyson официальный интернет магазин [url=https://dyson-kupit.com/]официальный сайт dyson в россии интернет магазин[/url] .
К-ЖБИ – завод, который предоставляет железобетонные изделия и удивляет ассортиментом. Выполняем быструю доставку и уделяем большое внимание качеству выпускаемой продукции. Ищете элемент приямка монолитный цельный? Gbisp.ru – сайт, где представлены контакты и наши реализованные проекты, ознакомиться с ними можете в любое удобное для вас время. Также здесь можно оставить заявку, просто укажите ваше имя, контактный номер телефона, e-mail, после этого нажмите на соответствующую кнопку «Отправить». Для уточнения информации обратитесь к опытному менеджеру.
Интернет магазин DinAvto.ru – это верное решение владельца авто. Мы все знаем о предоставляемых нами товарах. У нас вы приобретете то, что идеально подойдет вашей машине. Предлагаем большой выбор автозапчастей, цены у нас привлекательные. Ищете тормозная жидкость купить? Dinavto.ru – сайт, оплачиваете заказ здесь, а оплата за доставку производится вами при получении. Наша цель – удовлетворить ваши запросы. Товары доставляем по всей Рф, Москве и Московской области. Обращайтесь к нам прямо сейчас, готовы вас профессионально проконсультировать.
https://novostroykatoday.ru/ купить квартиру от застройщика в Казани с гарантией
На сайте https://ocims.ru/ закажите звонок для того, чтобы вам рассчитали стоимость каркасных домов в Орле. Они создаются по индивидуальному эскизу либо готовому проекту. На возведение уходит не более пары месяцев, а на сооружения дается гарантия 5 лет. В договоре прописывается стоимость, которая потом не изменяется. «ОЦИМС» гарантирует строительство комфортного, уютного дома, в котором вам точно понравится проживать. Каждый клиент сможет рассчитывать на приятный презент. Компания также занимается возведением домов в ипотеку от 5%.
LASTTORRENT.RU предлагает быстро скачать понравившуюся игру через торрент. Вы не будете скучать в ожидании. Удобный рубрикатор есть только у нас. Мы не загромождаем страницы ненужными баннерами, отыскать искомое можете без каких-либо усилий. Вам необязательно проходить регистрацию для доступа к файлам торрентов. Ищете игры на выживание? Lasttorent.ru – сайт бесплатный, при скачивании игры, здесь вы получите исправный продукт без угрозы заражения вирусами своего компьютера. В случае возникших вопросов, смело обращайтесь к нам. Наслаждайтесь игровым процессом!
https://bvbagemori.com/
Сантехника в Москве от https://sanbest.ru это возможность купить по привлекательным ценам сантехнику в Москве, душевые кабины в Москве. Купить раковину Москва также не составит труда. Большой ассортимент, доставка, различные акции и распродажи помогут выгодно приобрести сантехнику. Подробнее на сайте.
вызвать шлюху москва
На сайте https://kreditbaza.ru/ ознакомьтесь со всеми предложениями по кредитам, займам, которые выдают в России. Перед вами самые лучшие предложения, которыми вы сможете воспользоваться прямо сейчас. Возьмите средства без процентов до 100 000 рублей. Все предприятия, которые находятся на сайте, есть в госреестре МФО. Каждый желающий сможет получить до 30 млн. рублей. Оформите кредитную карту по привлекательной ставке – 9%. Также есть дебетовые карты с кэшбэком до 30%. РКО предоставляется бесплатно.
Покупки станут дешевле – получи Кэшбэк https://maxpromokod.ru/ до 30%! У нас более 4 500 интернет-магазинов и 33 000 промокодов и акций скидок.
кодировка уколом от алкоголизма https://addictiontreatment.kz/
лечение наркомании нарколог https://narcohelp.kz/
Vavada
Relomania предоставляет исключительно высококачественные услуги. Компания специализируется на упрощении трудного процесса получения ВНЖ в Испании. Мы гарантируем отсутствие скрытых комиссий, цены наши прозрачны. Справимся с любой задачей, стремимся к совершенству. Ищете внж испании без права на работу? Relomania.com – сайт, где можно в удобное время оставить заявку на профессиональную консультацию. В ближайшее время свяжемся с вами. Уделим время на понятие вашей ситуации. Воплотите ваши стремления в реальность. Мы сделаем ваш переезд в Испанию комфортным, обращайтесь к нам!
zgrwo rnsln rwhjg mzdlk ppbui fjgun
Привет всем!
Рады предложить продажу и установку входных и межкомнатных дверей в Саратове и области. За время работы накоплен большой опыт для решения задач, связанных с установкой и сервисным обслуживанием дверных конструкций. Основными принципами в своей работе мы считаем обязательность, соблюдение договоренностей, выполнение оговоренных работ точно в срок, предсказуемо высокий уровень сервиса.
Мы предлагаем клиентам более 1000 моделей качественных входных и межкомнатных дверей различных по стоимости, материалу отделки, конструкции и другим параметрам от известных производителей. На связи с Вами всегда настоящие специалисты, знающие все тонкости и нюансы данной области. Благодаря этому, профессиональные консультанты помогут подобрать оптимальную конфигурацию, что будет залогом надежной, безотказной работы и комфорта.
Вся информация на сайте https://id64.ru/
Все заботы клиента мы берем на себя. Подбор двери, замер проемов, доставка, установка, обслуживание — полный комплекс услуг под ключ.
По желанию клиента, двери могут изготавливаться с индивидуальными параметрами: тип отделки, фурнитура, размеры.
Консультацию проводят высококвалифицированные мастера. Они помогут найти лучшее изделие, подходящее под все Ваши требования.
Вы можете купить у нас двери в рассрочку без переплат при участии банков: Сбер, Почта банк, МТС банк, Русский Стандарт, Хоум кредит, Халва.
Двери гармошка
Двери царговые
Входные двери
Двери в подъезд
Двери с терморазрывом
Двери в зал
Удачи и хорошего интерьера!
На сайте https://nnprom.ru/ вы сможете приобрести сварной решетчатый настил, прессованный решетчатый настил, а также ступени из настила, крепления для решетчатого настила, перфорированный лист. Компания занимается непосредственно производством конструкций. Для этого имеются все мощности, инновационное и высокотехнологичное оборудование. Производится более 100 тонн конструкций в месяц. При необходимости менеджеры разработают схемы на основании чертежей. Продукция отличается высоким качеством, создана в соответствии с ГОСТом, нормативами.
Компания «Мсмтепло» длительное время производит и реализует теплообменное оборудование в Москве и по привлекательной стоимости, различной сложности. Перед вами огромный выбор продукции, созданной для различных целей: строительных компаний, промышленности, бассейнов, а также ЖКХ, котельных автономного типа. На сайте https://msmteplo.ru/ ознакомьтесь с расценками и другой полезной информацией.
регистрация leebet
На сайте https://interpol-s.ru/ вы сможете заказать проверку на полиграфе. Вы пройдете тестирование на профессиональном устройстве. Проводятся зависимые и независимые экспертизы. Вся информация проверяется на достоверность. В обязательном порядке соблюдается закон, требования. Записаться на услугу можно в любое время, когда вам удобно. В компании оказываются такие популярные и важные услуги, как: проверка на полиграфе, полиграф для женщин, проведение судебных экспертиз, тестирование персонала.
Столбец бэклинков
После того как многочисленных обновлений поисковой системы G необходимо внедрять разные варианты ранжирования.
Сегодня есть способ привлечь внимание поисковым системам к вашему сайту с помощью бэклинков.
Обратные ссылки не только эффективный инструмент продвижения, но и имеют органический трафик, прямых продаж с этих ресурсов скорее всего не будет, но переходы будут, и именно поеденического трафика мы тоже получаем.
Что в итоге получим на выходе:
Мы отображаем сайт поисковым системам с помощью обратных ссылок.
Получают органические переходы на сайт, а это также сигнал поисковым системам о том, что ресурс используется людьми.
Как мы демонстрируем поисковым системам, что сайт ликвиден:
1 ссылка на главную страницу, где содержится основная информация, создается.
Размещаем обратные ссылки через редиректы с трастовых ресурсов.
Главное – мы добавляем сайт в специализированные инструменты для анализа сайтов, где он кэшируется, а затем полученные ссылки размещаются в виде редиректов на блогах, форумах, в комментариях.
Это нужное действие показывает всем поисковикамКАРТУ САЙТА, так как анализаторы сайтов отображают всю информацию о сайтах со всеми ключевыми словами и заголовками и это очень ХОРОШО
uqrlp xuegh ynrnn ncjyg yaqlm bwrav
Ищете новые анонсы серверов lineage 2? LA2-ANONS.COM – то, что вам нужно. Дадим вам грамотный совет в подборе надежного игрового сервера. На нашем портале анонсы серверов Lineage 2 регулярно актуализируются, это позволяет любому геймеру быть первым зарегистрированным. Интересует lineage 2? La2-anons.com – сайт, мы каждый день работаем над его функционалом. Обратите свое внимание на платформы с пометкой VIP, при выборе сервера для игры Lineage 2. Предлагаем только актуальную информацию, с которой вы можете прямо сейчас ознакомиться.
https://novostroyzhkspb.ru/
купить обучение
права на автовышку
Дайсон [url=http://www.dyson-kupit.com]Дайсон[/url] .
Хотите купить по недорогой стоимости надежные и функциональные шлагбаумы? Вы точно попали по адресу! У нас есть шлагбаумы любой стоимости. Ассортимент наш очень большой, мы вас приятно удивим. Предлагаем только сертифицированную продукцию. Ищете установка шлагбаума? Senator-sb.ru – сайт, где вы можете приобрести шлагбаум и сделать заказ на установку конструкции на вашем объекте. Оформить заявку либо уточнить актуальную стоимость товаров, можно связавшись с нами по контактному номеру телефона. На вашей территории обеспечьте безопасность уже сейчас!
ремонт ноутбука в москве
ремонт телефонов рядом
ремонт сотовых телефонов в москве
На сайте https://1roma.ru/ вы сможете выбрать и заказать такой строительный материал, как: фасадные панели, сайдинг, дренажные системы, водостоки, газонные решетки и многое другое. Перед вами огромный ассортимент вариантов, привлекательных коллекций различных фактур и цветовых решений. Несколько сотен вариантов отделки, в том числе, темные цветостойкие панели. Прямо сейчас посмотрите все доступные варианты. Вы сможете пролистать каталог всего за пару минут, чтобы ознакомиться с готовыми решениями.
На сайте https://bluedroid.ru/ игры и приложения для Андроид телефона, читы, приватные сервера и пользовательские модификации игр с убранной рекламой и неограниченной валютой!
ycoua ledlr ghpob waqog gjvhq nskak
Ищите, где бесплатно, оперативно и без лишних проблем скачать интересующую игру? ТОРРЕНТ-ГЕЙМ предоставляет такую прекрасную возможность. Вам необходимо выполнить установку программы на ПК – Torrent. Под любую игру имеется отдельная страница сайта, на ней – скриншоты прохождения, подробные описания. Ищете скачать игры по сети через торрент? Torrent-game.net – сервис, где вы найдете то, что вас порадует. Увлекательный виртуальный мир ждет вас. Отвлекитесь от рабочей рутины и повседневного быта. Прекрасного вам игрового процесса!
GAMETORRENT.NET – ресурс, где вы найдете все нужное для погружения в захватывающий мир игр. Мы предлагаем большой выбор разных жанров. У нас есть много аркад и головоломок для разминки мозга, также имеются ролевые игры, шутеры и многое другое. Даем гарантию на то, что тут вы найдете то, что вам по душе. Ищете скачать шутеры на пк через торрент? Gametorrent.net – сайт, где вас ожидают свежие новинки игровой индустрии. Не сомневайтесь в том, что получите только лучшие игры без вредоносных программ и вирусов. Окунитесь в мир неимоверных возможностей, скачивайте игры уже сейчас!
dysons-shop.ru [url=dyson-kupit.com]dyson официальный интернет магазин[/url] .
Dyson [url=https://dyson-kupit.com/]Dyson[/url] .
dyson официальный магазин [url=dyson-kupit.com]dyson официальный сайт интернет магазин[/url] .
На сайте https://beperfect-shop.ru/ вы приобретете качественные материалы для наращивания, а также ламинирования ресниц. Вся продукция является качественной, безопасной и функциональной. Регулярно проходят распродажи, на отдельные группы товаров действуют скидки. В разделе находится клей, ресницы, а также дополнительные материалы, которые потребуются для профессионального оказания услуги. В интернет-магазине постоянно появляются новинки, которые применяются в салонах красоты.
https://irongamers.ru/sale/
На сайте https://stimul57.ru/ сделайте заказ для того, чтобы приобрести дорожные знаки напрямую от производителя. К важным преимуществам компании относят то, что у нее собственное производство, практичные крепления, а на всю продукцию предоставляются гарантии 7 лет. В работе применяются материалы высокого качества, сертифицированные. Организуется доставка по всей России. Все товары полностью отвечают техническому регламенту, требованиям. На всех этапах производства проходят контроль качества.
Дорожная служба спасения 911 с успехом оказывает услуги в области эвакуации легкового и грузового транспорта. Гарантируем вам профессионализм всех сотрудников и оперативность. Ищете грузовой эвакуатор? Penza-evakuator.ru – сайт, где можете прямо сейчас посмотреть фото нашей работы и оставить заявку. Для этого укажите ваше имя и контактный номер телефона. Здесь можете ознакомиться с отзывами довольных клиентов и прайс-листом. Также вы детальнее узнаете, какие мы готовы эвакуировать виды техники. Обращайтесь именно к нам, будем рады вам помочь!
Хотите смотреть увлекательные фильмы и сериалы совершенно бесплатно в хорошем качестве? Lords.lat – это то, что вам нужно! Здесь представлены фильмы с захватывающим сюжетом. Драмы, комедии, фантастика, ужасы, детективы, боевики – все это доступно для вас. Отправляйтесь в чудесный мир, полный приключений. https://2024-films.lords.lat – сайт, где можете найти то, что пожелаете. Здесь вы отыщите поиск, который можно в любой момент применить. Не упустите возможность насладиться просмотром фильмов, сериалов и мультфильмов в великолепном качестве!
На сайте https://www.prokinvest.ru/ вы сможете купить квартиру в СПб от застройщика ГК ПРОК, готовые квартиры в новостройках и в строящихся домах. Зайдите на сайт, ознакомьтесь с вариантами, условиями покупки, акциями застройщика и выберите для себя идеальный вариант.
Квартиры в Екатеринбурге https://newflatekb.ru/ купить от официального застройщика
Came across an interesting article, worth a glance https://rodme.ru/oschutite-adrenalin-i-azart-igry-na-betunlim-luchshee-mesto-dlya-pobediteley-t11993.html
Player線上娛樂城遊戲指南與評測
台灣最佳線上娛樂城遊戲的終極指南!我們提供專業評測,分析熱門老虎機、百家樂、棋牌及其他賭博遊戲。從遊戲規則、策略到選擇最佳娛樂城,我們全方位覆蓋,協助您更安全的遊玩。
Player如何評測:公正與專業的評分標準
在【Player娛樂城遊戲評測網】我們致力於為玩家提供最公正、最專業的娛樂城評測。我們的評測過程涵蓋多個關鍵領域,旨在確保玩家獲得可靠且全面的信息。以下是我們評測娛樂城的主要步驟:
娛樂城是什麼?
娛樂城是什麼?娛樂城是台灣對於線上賭場的特別稱呼,線上賭場分為幾種:現金版、信用版、手機娛樂城(娛樂城APP),一般來說,台灣人在稱娛樂城時,是指現金版線上賭場。
線上賭場在別的國家也有別的名稱,美國 – Casino, Gambling、中國 – 线上赌场,娱乐城、日本 – オンラインカジノ、越南 – Nhà cái。
娛樂城會被抓嗎?
在台灣,根據刑法第266條,不論是實體或線上賭博,參與賭博的行為可處最高5萬元罰金。而根據刑法第268條,為賭博提供場所並意圖營利的行為,可能面臨3年以下有期徒刑及最高9萬元罰金。一般賭客若被抓到,通常被視為輕微罪行,原則上不會被判處監禁。
信用版娛樂城是什麼?
信用版娛樂城是一種線上賭博平台,其中的賭博活動不是直接以現金進行交易,而是基於信用系統。在這種模式下,玩家在進行賭博時使用虛擬的信用點數或籌碼,這些點數或籌碼代表了一定的貨幣價值,但實際的金錢交易會在賭博活動結束後進行結算。
現金版娛樂城是什麼?
現金版娛樂城是一種線上博弈平台,其中玩家使用實際的金錢進行賭博活動。玩家需要先存入真實貨幣,這些資金轉化為平台上的遊戲籌碼或信用,用於參與各種賭場遊戲。當玩家贏得賭局時,他們可以將這些籌碼或信用兌換回現金。
娛樂城體驗金是什麼?
娛樂城體驗金是娛樂場所為新客戶提供的一種免費遊玩資金,允許玩家在不需要自己投入任何資金的情況下,可以進行各類遊戲的娛樂城試玩。這種體驗金的數額一般介於100元到1,000元之間,且對於如何使用這些體驗金以達到提款條件,各家娛樂城設有不同的規則。
娛樂城評價
Player線上娛樂城遊戲指南與評測
台灣最佳線上娛樂城遊戲的終極指南!我們提供專業評測,分析熱門老虎機、百家樂、棋牌及其他賭博遊戲。從遊戲規則、策略到選擇最佳娛樂城,我們全方位覆蓋,協助您更安全的遊玩。
Player如何評測:公正與專業的評分標準
在【Player娛樂城遊戲評測網】我們致力於為玩家提供最公正、最專業的娛樂城評測。我們的評測過程涵蓋多個關鍵領域,旨在確保玩家獲得可靠且全面的信息。以下是我們評測娛樂城的主要步驟:
娛樂城是什麼?
娛樂城是什麼?娛樂城是台灣對於線上賭場的特別稱呼,線上賭場分為幾種:現金版、信用版、手機娛樂城(娛樂城APP),一般來說,台灣人在稱娛樂城時,是指現金版線上賭場。
線上賭場在別的國家也有別的名稱,美國 – Casino, Gambling、中國 – 线上赌场,娱乐城、日本 – オンラインカジノ、越南 – Nhà cái。
娛樂城會被抓嗎?
在台灣,根據刑法第266條,不論是實體或線上賭博,參與賭博的行為可處最高5萬元罰金。而根據刑法第268條,為賭博提供場所並意圖營利的行為,可能面臨3年以下有期徒刑及最高9萬元罰金。一般賭客若被抓到,通常被視為輕微罪行,原則上不會被判處監禁。
信用版娛樂城是什麼?
信用版娛樂城是一種線上賭博平台,其中的賭博活動不是直接以現金進行交易,而是基於信用系統。在這種模式下,玩家在進行賭博時使用虛擬的信用點數或籌碼,這些點數或籌碼代表了一定的貨幣價值,但實際的金錢交易會在賭博活動結束後進行結算。
現金版娛樂城是什麼?
現金版娛樂城是一種線上博弈平台,其中玩家使用實際的金錢進行賭博活動。玩家需要先存入真實貨幣,這些資金轉化為平台上的遊戲籌碼或信用,用於參與各種賭場遊戲。當玩家贏得賭局時,他們可以將這些籌碼或信用兌換回現金。
娛樂城體驗金是什麼?
娛樂城體驗金是娛樂場所為新客戶提供的一種免費遊玩資金,允許玩家在不需要自己投入任何資金的情況下,可以進行各類遊戲的娛樂城試玩。這種體驗金的數額一般介於100元到1,000元之間,且對於如何使用這些體驗金以達到提款條件,各家娛樂城設有不同的規則。
Рады вас приветствовать на BDA Logistic! Мы специализируемся на доставке товаров из Китая в РФ и предлагаем комплексное решение для продавцов на маркетплейсах. Работаем с физическими и юридическими лицами. https://bdalogistic.ru – здесь вы узнаете, как осуществляется оплата, можно ли застраховать груз, как проверяется качество товара. Также у вас есть возможность тут получить индивидуальное предложение и оставить заявку. Для этого в специальную форму на сайте необходимо ввести ваше имя и контактный номер телефона, после чего нажать на кнопку «Отправить».
Курсовые и дипломные работы https://newflatekb.ru/ на заказ. Выполняем любые типы работ онлайн в короткие сроки по выгодным ценам для студентов.
Tikstroy.ru – сайт, который предлагает недорогие услуги по укладке тротуарной плитки. Профессиональные мастера своего дела выполняют работы качественно и в короткие сроки. Доверьтесь специалистам. Ищете укладка тротуарной плитки под ключ в серпухове? Tikstroy.ru – сайт, где можно прямо сейчас посмотреть наши некоторые работы. Гарантируем индивидуальный подход к каждому клиенту, прислушиваемся ко всем замечаниям и пожеланиям. С радостью вас проконсультируем по всем вопросам. Обращайтесь к нам, полностью удовлетворим ваши потребности.
сколько стоит дайсон в дубае [url=http://dyson-2024.com/]dyson airwrap цена[/url] .
https://satbayev.university/
Discovered an article that might catch your interest – don’t miss it! http://forum.golebieonline.pl/viewtopic.php?f=6&t=375547
Discovered an article that will surely interest you – I recommend checking it out http://doremichildcarecentre.com/index.php/forum/welcome-mat/191302
Почему теневой плинтус – красивая и практичная деталь интерьера,
Как правильно установить теневой плинтус своими руками,
Теневой плинтус как элемент декора: идеи и варианты применения,
Модные тренды в выборе теневых плинтусов для современного дома,
Советы стилиста: как сделать цвет теневого плинтуса акцентом в помещении,
Безопасность и стиль: почему теневой плинтус – идеальное решение для дома,
Теневой плинтус с подсветкой: создаем эффектное освещение в интерьере,
Как сделать помещение завершенным с помощью теневого плинтуса,
Почему теневой плинтус – важная деталь в оформлении интерьера
теневой плинтус стоимость [url=https://plintus-tenevoj-aljuminievyj-msk.ru/]https://plintus-tenevoj-aljuminievyj-msk.ru/[/url] .
Я давно мечтал повысить свою квалификацию и пройти профессиональные курсы. Но как назло, на это не хватало средств. Открыв телеграм-канал [url=https://t.me/s/zaim_0_procent/]Займы без процентов на карту[/url] , я нашел несколько предложений, идеально подходящих для моей ситуации. Через пару часов деньги были у меня на карте, и я смог оплатить обучение. Эти курсы открыли передо мной новые карьерные возможности.
Stumbled upon an interesting article – I suggest you take a look https://www.prideofdetroit.com/users/cchatruletka
Found an enthralling read that I’d recommend – it’s truly fascinating http://g95334gq.beget.tech/2024/03/10/onlayn-ploschadka-dlya-romanticheskih-znakomstv-s-devushkami.html
Мне нужно было срочно купить новый телефон. Моя кредитная история оставляла желать лучшего, но на [url=https://t.me/s/new_mfo_2024/]новые МФО 2024 без отказа[/url] я нашел подборку займов для людей с плохой кредитной историей. Выбрал один из них и получил деньги буквально за считанные минуты. Телефон был куплен в тот же день.
купить подписчиков вк
купить просмотры тик ток
Came across a unique piece – be sure to check it out https://krasnevikno.com.ua/trudovoj-den-vodytelya-taksy-rasporyadok-y-soveti/
https://smm.ytmonster.ru/telegram/likes/nakrutka-reaktsii-telegram
виды кондиционеров
https://onlinecrashgame.space/bg/jetx/
https://vyzvat-taksi.ru/
индивидуальный подход к каждому дому.
проекты домов из газобетона [url=http://planirovkidomov24.ru/catalog/doma-iz-gazobetona/]http://planirovkidomov24.ru/catalog/doma-iz-gazobetona/[/url] .
Привет!
Жизнь Винницы в новостях: последние события, главные новости и самые интересные истории. Оставайтесь с нами, чтобы знать все о своем городе.
Все самое лучшее на сайте https://bomba.vn.ua/
[url=https://bomba.vn.ua/category/novini-ukraini/]Новости Украины за день[/url]
Новости Винницы сегодня
последние новости Винницы
Новости Винницы фото
Удачи!
Cataract treatment at MCI Clinic involves a modern approach to restoring vision. Our specialists use the latest methods, including ultrasonic phacoemulsification. This minimally invasive procedure allows for the removal of the cloudy lens and its replacement with an artificial one. The operation takes only a few minutes and is performed under local anesthesia, ensuring a comfortable experience for the patient.
Choosing MCI for cataract treatment means trusting experienced professionals dedicated to your eye health. Post-operative care includes regular check-ups to ensure optimal recovery and long-lasting results. Our goal is to help you regain clear vision and improve your quality of life.
MCI Clinic – [url=https://mci.md/]astigmatizm[/url]
Замена венцов красноярск
Gerakl24: Квалифицированная Замена Основания, Венцов, Покрытий и Передвижение Строений
Компания Геракл24 профессионально занимается на предоставлении всесторонних работ по смене основания, венцов, полов и перемещению домов в населённом пункте Красноярском регионе и в окрестностях. Наша группа квалифицированных экспертов обеспечивает высокое качество исполнения всех видов ремонтных работ, будь то деревянные, с каркасом, кирпичные постройки или бетонные конструкции дома.
Достоинства услуг Gerakl24
Навыки и знания:
Каждая задача выполняются исключительно высококвалифицированными специалистами, с обладанием большой стаж в области возведения и восстановления строений. Наши специалисты профессионалы в своем деле и осуществляют работу с высочайшей точностью и вниманием к деталям.
Комплексный подход:
Мы предлагаем полный спектр услуг по ремонту и восстановлению зданий:
Смена основания: усиление и реставрация старого основания, что позволяет продлить срок службы вашего здания и предотвратить проблемы, вызванные оседанием и деформацией.
Реставрация венцов: замена нижних венцов деревянных домов, которые чаще всего подвергаются гниению и разрушению.
Установка новых покрытий: монтаж новых настилов, что значительно улучшает визуальное восприятие и функциональные характеристики.
Перемещение зданий: безопасное и надежное перемещение зданий на новые места, что обеспечивает сохранение строения и избегает дополнительных затрат на создание нового.
Работа с любыми типами домов:
Деревянные дома: реставрация и усиление деревянных элементов, защита от гниения и вредителей.
Каркасные строения: усиление каркасных конструкций и замена поврежденных элементов.
Дома из кирпича: ремонт кирпичных стен и усиление стен.
Бетонные строения: реставрация и усиление бетонных элементов, исправление трещин и разрушений.
Надежность и долговечность:
Мы применяем лишь качественные материалы и новейшее оборудование, что гарантирует долговечность и прочность всех выполненных задач. Все проекты подвергаются строгому контролю качества на каждом этапе выполнения.
Личный подход:
Мы предлагаем каждому клиенту индивидуальные решения, учитывающие ваши требования и желания. Мы стараемся, чтобы результат нашей работы полностью удовлетворял ваши ожидания и требования.
Почему выбирают Геракл24?
Обратившись к нам, вы получаете надежного партнера, который берет на себя все заботы по ремонту и реставрации вашего дома. Мы обещаем выполнение всех работ в установленные сроки и с в соответствии с нормами и стандартами. Обратившись в Геракл24, вы можете быть спокойны, что ваш дом в надежных руках.
Мы готовы предоставить консультацию и ответить на все ваши вопросы. Звоните нам, чтобы обсудить ваш проект и узнать больше о наших услугах. Мы сохраним и улучшим ваш дом, сделав его уютным и безопасным для долгого проживания.
Геракл24 – ваш выбор для реставрации и ремонта домов в Красноярске и области.
Доброго!
Новости Украины и мира: читайте о главных событиях дня, важных международных новостях и их влиянии на нашу страну. Будьте информированы обо всем.
Все самое лучшее на сайте https://omind.com.ua/category/tech/
[url=https://omind.com.ua/]Новости Украины сегодня[/url]
новости Украины видео
новости Украины за день
последние новости мира
Удачи!
качественная заливка бетона https://betonzavody.ru/
сео продвижение сайта
Transform your vision with cataract surgery at MCI Clinic! Our experienced surgeons utilize advanced phacoemulsification techniques to remove cloudy lenses and replace them with high-quality artificial ones. This minimally invasive procedure is quick, safe, and ensures a remarkable improvement in your vision. At MCI, we combine expertise, cutting-edge equipment, and affordable pricing to provide you with the best possible care.
Choose MCI Clinic for your cataract surgery and see the world in stunning clarity. Experience the difference of top-tier medical care and regain the vibrant vision you deserve.
MCI Clinic – [url=https://mci.md/]miopie[/url]
seo оптимизация
Ищете способ быстро получить деньги? Подпишитесь на наш телеграм-канал [url=https://t.me/s/MFO_dayt_vsem/]займеры которые дают всем[/url] ! Здесь мы собрали лучшие МФО, которые выдают деньги без отказов и проверки кредитной истории. Первый займ под 0% до 15 000 рублей всего за 10 минут! В канале также есть подборки новых МФО 2024 года, выдающих микрозаймы мгновенно, даже если у вас плохая кредитная история или просрочки. Присоединяйтесь и решайте финансовые вопросы легко!
https://eroticahd.ru/
Доброго!
Транспортная сеть Одессы обновляется и расширяется. Ознакомьтесь с проектами, которые сделают передвижение по городу более удобным и безопасным.
Все самое лучшее на сайте https://bomba.od.ua/category/novini-odesi/
[url=https://bomba.od.ua/category/novini-ukraini/]Новости Украины ТОП[/url]
новости Украины видео
Главные новости Одессы
Новости Одессы за день
Удачи!
https://topthuthuat.com/top-5-trang-web-ung-dung-pho-bien-nhat-o-viet-nam-bi-quyet-kiem-tien-qua-proxy/
Found captivating reading that I’d like to offer you – you won’t regret it https://www.blazersedge.com/users/cchatruletka
купить спортивный велосипед https://velo4u.ru/
Discovered an article that might catch your interest – don’t miss it! https://onefootdown.com/users/cchatruletka
1вин казино
Соут заказать Москва safetysystemsgroup.com
Компания Safety Systems проводит спец оценку условий труда на каждом предприятии. Наш основной офис расположен в Москве, но ещё в большинстве регионов РФ у нас есть филиалы. Проверка проводится в обязательном порядке для всех организаций, чтобы снабдить безопасность для сотрудников и сократить шансы на получение штрафов.
Относительно [url=https://safetysystemsgroup.com/sout3/]экспертный центр соут[/url] заходите на наш сайт. Оценочные мероприятие должны осуществляться в организациях не реже, чем раз в 5 лет. Но также у особых отраслей есть индивидуальные сроки. Обязательно ознакомьтесь с тем, что нужно конкретно Вам на сайте safetysystemsgroup.com прямо сейчас.
Здравствуйте!
Транспортные изменения в Хмельницком: Городская инфраструктура обновляется, включая новые маршруты общественного транспорта и улучшение дорог. Узнайте, как эти изменения повлияют на передвижение по городу.
Все самое лучшее на сайте https://bomba.km.ua/
[url=https://bomba.km.ua/]Новости Хмельницкого за день[/url]
Новости Украины лента
последние новости Украины
Новости Хмельницкого аварии
Удачи!
купить диплом вуза [url=https://kupitediplom0029.ru]https://kupitediplom0029.ru[/url] .
https://arochnye-shatry.ru/
Экспертиза соут safetysystemsgroup.com
Фирма Safety Systems реализует специальную оценку условий труда на каждом предприятии. Наш основной офис находится в Москве, но ещё в большинстве регионов России у нас есть филиалы. Проверка осуществляется в обязательном порядке для всех компаний, чтобы обеспечить безопасность для сотрудников и убавить шансы на получение штрафов.
От нас [url=https://safetysystemsgroup.com/sout3/]аккредитованные организации по специальной оценке условий труда[/url] – от Вас звонок в нашу фирму. Расположены по адресу: 105264, г. Москва, ул. Верхняя Первомайская, д. 47, к. 11, оф. 516. У нас собственная аккредитованная лаборатория, которая поможет Вам сэкономить до 80 % от конкурентной стоимости. Звоните, обращайтесь и заказывайте соут.
Официальный центр оформления и выпуска международных водительских прав (удостоверений) МВУ в Казахстане. Международные права с онлайн оформлением, с бесплатной доставкой.
Came across a unique piece Ц be sure to check it out http://bmc.ukrbb.net/viewtopic.php?f=8&t=13165
Ваша посудомоечная машина Bosch нуждается в ремонте? Наши специалисты с более чем 10-летним опытом предоставляют профессиональный [url=https://xn—-9sbn2afcdnw7c.xn--p1ai/]ремонт посудомоечных машин Bosch[/url] с использованием оригинальных запчастей. Бесплатный выезд мастера и оперативное устранение любых неисправностей.Ваша посудомоечная машина Bosch не работает как раньше? Наши специалисты готовы выполнить [url=https://xn—-9sbn2afcdnw7c.xn--p1ai/]ремонт посудомоечных машин Bosch[/url] быстро и надежно. Мы используем только оригинальные запчасти и предоставляем гарантию на все виды работ.
купить диплом бакалавра https://kupitediplom0029.ru
Discovered an article that will surely interest you – I recommend checking it out https://imgur.com/user/worksale/about
Наш [url=https://xn—-9sbn2afcdnw7c.xn--p1ai/]Бош центр[/url] в Москве предлагает лучшие условия для ремонта бытовой техники. Бесплатная диагностика, ремонт на дому и гарантия на 1 год — все это обеспечивает высокий уровень сервиса и удовлетворение клиентов.Ваш холодильник Бош не работает? Наши мастера с более чем 10-летним опытом проведут [url=https://xn—-9sbn2afcdnw7c.xn--p1ai/]ремонт холодильников Бош[/url] на дому. Мы используем только оригинальные запчасти и предлагаем бесплатный выезд мастера для диагностики и устранения проблем.
Здравствуйте!
Экологические инициативы в Украине: Как Украина борется с загрязнением окружающей среды и какие проекты направлены на улучшение экологической ситуации в стране.
Все самое лучшее на сайте https://beta.in.ua/sport/
[url=https://beta.in.ua/ekonomika/]Новости Экономики[/url]
последние новости мира
новости мира
новости Украины видео
Удачи!
автовыкуп дорого [url=https://www.vykup-avtomsk.ru]автовыкуп дорого[/url] .
Клининговая компания после умершего https://prof-uborka-posle-smerti.ru/
швеллер размеры гост цены
https://www.datafilehost.com/the-art-of-anonymity-how-proxy-vpns-protect-your-digital-identity
сеть сайтов pbn
Работая в сео, нужно знать, что нельзя одним инструментом вывести сайт в топ поисковой выдачи поисковиков, поскольку системы поиска это как дорожка с финишной прямой, а сайты это гоночные автомобили, которые все стремятся быть первыми.
Так вот:
Перечень – Для того чтобы сайт был адаптивен и быстр, важна
оптимизация
Сайт должен содержать только уникальное содержимое, это тексты и картинки
В ОБЯЗАТЕЛЬНОМ ПОРЯДКЕ ссылочная масса через сайты с статьями и напрямую на главную страницу
Увеличение обратных ссылок с применением второстепенных сайтов
Пирамида ссылок, эти ссылки первого уровня, Tier-2, третьего уровня
И самое главное это сеть сайтов PBN, которая ссылается на основной сайт
Все сайты PBN должны быть без следов, т.е. поисковики не должны понимать, что это один владелец всех веб-сайтов, поэтому ОЧЕНЬ важно следовать все эти указания.
печать визиток
Came across an interesting article, worth a glance http://kolba.com.ua/index.php?topic=139566.new#new
https://jasdam.cz
Discovered an article that will definitely interest you – don’t miss the chance to familiarize yourself https://shounen.ru/ekonomika/3475-vlijanie-jeskort-uslug-na-obschestvo-i-individualnye-otnoshenija.html
在線娛樂城的世界
隨著互聯網的迅速發展,在線娛樂城(線上賭場)已經成為許多人娛樂的新選擇。在線娛樂城不僅提供多樣化的游戲選擇,還能讓玩家在家中就能體驗到賭場的樂趣和樂趣。本文將探討線上娛樂城的特色、利益以及一些常見的的遊戲。
什麼叫在線娛樂城?
線上娛樂城是一種經由互聯網提供賭錢游戲的平台。玩家可以通過電腦、手機或平板電腦進入這些網站,參與各種賭博活動,如撲克、賭盤、二十一點和吃角子老虎等。這些平台通常由專業的程序公司開發,確保游戲的公平性和安全性。
網上娛樂城的利益
方便性:玩家不需要離開家,就能享用賭博的快感。這對於那些居住在遠離的實體賭場地區的人來說尤其方便。
多樣化的游戲選擇:線上娛樂城通常提供比實體賭場更多的游戲選擇,並且經常改進游戲內容,保持新穎。
福利和獎勵計劃:許多線上娛樂城提供多樣的獎金計劃,包括註冊紅利、存款獎勵和忠誠度計劃,引誘新玩家並鼓勵老玩家不斷遊戲。
穩定性和保密性:正規的線上娛樂城使用先進的加密技術來保護玩家的私人信息和交易,確保遊戲過程的穩定和公正。
常見的在線娛樂城游戲
德州撲克:德州撲克是最受歡迎博彩遊戲之一。線上娛樂城提供各種德州撲克變體,如德州撲克、奧馬哈撲克和七張牌等。
輪盤:輪盤是一種古老的賭場遊戲遊戲,玩家可以下注在數字、數字排列或顏色上,然後看轉球落在哪個地方。
21點:又稱為黑傑克,這是一種對比玩家和莊家點數的遊戲,目標是讓手牌點數盡可能接近21點但不超過。
老虎機:老虎機是最簡單並且是最受歡迎的賭博游戲之一,玩家只需旋轉捲軸,看圖案排列出贏得的組合。
結尾
網上娛樂城為現代賭博愛好者提供了一個方便、刺激的且豐富的娛樂方式。無論是撲克迷還是老虎機迷,大家都能在這些平台上找到適合自己的遊戲。同時,隨著技術的的不斷提升,網上娛樂城的游戲體驗將變得越來越越來越真實和有趣。然而,玩家在體驗游戲的同時,也應該保持自律,避免過度沉迷於賭博活動,維持健康的娛樂心態。
Наша компания предоставляет кредиты и займы в короткий срок, помогая клиентам решать финансовые вопросы быстро и без лишних хлопот, узнайте подробнее тут – https://hi-android.net/instrumenty/236073-debetovaya-karta-kak-rabotaet-zachem-nuzhna. Мы предлагаем простое и удобное оформление, минимальные требования к документам и мгновенное одобрение заявок.
娛樂城
網上娛樂城的世界
隨著互聯網的飛速發展,在線娛樂城(線上賭場)已經成為許多人消遣的新選擇。在線娛樂城不僅提供多種的游戲選擇,還能讓玩家在家中就能體驗到賭場的刺激和快感。本文將探討線上娛樂城的特色、優勢以及一些常見的遊戲。
什麼網上娛樂城?
在線娛樂城是一種經由互聯網提供賭博遊戲的平台。玩家可以通過計算機、智能手機或平板進入這些網站,參與各種賭博活動,如撲克、賭盤、黑傑克和老虎机等。這些平台通常由專家的程序公司開發,確保遊戲的公平性和安全性。
線上娛樂城的好處
便利:玩家不用離開家,就能享受賭博的樂趣。這對於那些生活在遠離實體賭場區域的人來說特別方便。
多樣化的遊戲選擇:線上娛樂城通常提供比實體賭場更多的遊戲選擇,並且經常升級遊戲內容,保持新鮮感。
福利和獎金:許多線上娛樂城提供豐富的優惠計劃,包括註冊紅利、存款獎金和忠誠度計劃,引誘新玩家並促使老玩家持續遊戲。
穩定性和保密性:正當的在線娛樂城使用高端的加密技術來保護玩家的私人信息和財務交易,確保遊戲過程的穩定和公正性。
常有的在線娛樂城游戲
撲克:撲克牌是最受歡迎賭錢遊戲之一。在線娛樂城提供多樣德州撲克變體,如德州撲克、奧馬哈撲克和七張撲克等。
輪盤賭:賭盤是一種經典的賭場遊戲遊戲,玩家可以賭注在數字、數字組合或顏色上,然後看小球落在哪個區域。
黑傑克:又稱為21點,這是一種對比玩家和莊家點數的游戲,目標是讓手牌點數盡量接近21點但不超過。
老虎机:老虎機是最容易且是最流行的賭博游戲之一,玩家只需旋轉捲軸,看圖案排列出獲勝的組合。
結論
網上娛樂城為現代的賭博愛好者提供了一個方便的、興奮且多樣化的娛樂活動。無論是撲克牌愛好者還是老虎機迷,大家都能在這些平台上找到適合自己的遊戲。同時,隨著技術的不斷提升,在線娛樂城的遊戲體驗將變化越來越逼真和引人入勝。然而,玩家在享用遊戲的同時,也應該自律,避免沉溺於博彩活動,保持健康的娛樂心態。
https://mikrocz.cz/
курсовые работы на заказ цены https://kontrolnyeaudit.ru/
лизинг без первоначального взноса для юридических лиц https://leasingber.ru
buy tiktok likes and views cheap buy tiktok views
Лучшие способы удаления бородавок на практике.
Кислота от бородавок [url=http://www.plastica.onclinic.ru/]http://www.plastica.onclinic.ru/[/url] .
Что делать, если вы обнаружили бородавку?
Плоские бородавки на руках [url=https://smclinic.ru]https://smclinic.ru[/url] .
Found a captivating read that I’d like to recommend to you https://tyachiv.ukraine7.com/login
Недавно у меня возникла непредвиденная ситуация — нужно было срочно оплатить медицинские услуги. Сумма составляла 20 тысяч рублей, а кредитная история у меня была не самой лучшей. На помощь пришёл канал https://t.me/s/novue_zaimu, где я нашёл новые займы на карту онлайн. Оказалось, что даже с плохой кредитной историей можно получить займ. Я воспользовался советами по выбору МФО и заполнению заявки, и через короткое время деньги были у меня на карте. Медицинские услуги были оплачены вовремя, и я смог решить свою проблему.
https://formomebel.ru/stoliki/metallicheskie
Доставка цветов в Саратове https://flowers64.ru/ это отличная возможность заказать различные цветы, букеты, композиции, подарки, не выходя из дома. Служба доставки работает круглосуточно, а сама доставка в течении 90 минут. Вы сможете подарить букет анонимно, просто напишите это при заказе. Огромный ассортимент цветов порадует всех!
заказать написание курсовой работы https://kontrolnyeaudit.ru/
Клининговая компания после пожара https://uborka-posle-pozhara-moskva.ru/
Незаменимая часть гардероба – тактичные штаны, дадут комфорт и уверенность.
Отличный выбор для походов и путешествий, тактичные штаны станут вашим надежным помощником.
Высокое качество и непревзойденный комфорт, сделают тактичные штаны незаменимым атрибутом вашего образа.
Современный стиль и практичность, подчеркнут вашу индивидуальность и статус.
Выберите качественные тактичные штаны, которые подчеркнут вашу силу и уверенность.
купити тактичні штани піксель [url=https://taktichmishtanu.kiev.ua/]https://taktichmishtanu.kiev.ua/[/url] .
Подземная канализация купить neseptik.com
Приобрести лучший септик можно нажав на страницу – [url=https://neseptik.com/]емкость под канализацию 5[/url] прямо сегодня. Звоните по следующему номеру телефона +7(902)871-48-01 и мы ответим на любые ваши вопросы. Находимся по адресу: г. Екатеринбург, ул. Вилонова, д. 33. График работы с пн по пт с 8:00 до 19:00. Звоните, приезжайте, мы будем рады сотрудничать с Вами!
Детективное агентство в Санкт-Петербурге. http://detektivnoe-agenstvo.ru/
sapporo88
Daily bonuses
Uncover Exciting Deals and Free Spins: Your Definitive Guide
At our gaming platform, we are devoted to providing you with the best gaming experience possible. Our range of offers and bonus spins ensures that every player has the chance to enhance their gameplay and increase their chances of winning. Here’s how you can take advantage of our incredible promotions and what makes them so special.
Lavish Free Spins and Rebate Promotions
One of our standout offers is the opportunity to earn up to 200 bonus spins and a 75% refund with a deposit of just $20 or more. And during happy hour, you can unlock this bonus with a deposit starting from just $10. This amazing offer allows you to enjoy extended playtime and more opportunities to win without breaking the bank.
Boost Your Balance with Deposit Promotions
We offer several deposit bonuses designed to maximize your gaming potential. For instance, you can get a free $20 promotion with minimal wagering requirements. This means you can start playing with extra funds, giving you more chances to explore our vast array of games and win big. Additionally, there’s a $10 deposit promotion available, perfect for those looking to get more value from their deposits.
Multiply Your Deposits for Bigger Wins
Our “Play Big!” offers allow you to double or triple your deposits, significantly boosting your balance. Whether you choose to multiply your deposit by 2 or 3 times, these offers provide you with a substantial amount of extra funds to enjoy. This means more playtime, more excitement, and more chances to hit those big wins.
Exciting Bonus Spins on Popular Games
We also offer up to 1000 bonus spins per deposit on some of the most popular games in the industry. Games like Starburst, Twin Spin, Space Wars 2, Koi Princess, and Dead or Alive 2 come with their own unique features and thrilling gameplay. These bonus spins not only extend your playtime but also give you the opportunity to explore different games and find your favorites without any additional cost.
Why Choose Our Platform?
Our platform stands out due to its user-friendly interface, secure transactions, and a wide variety of games. We prioritize your gaming experience by ensuring that all our promotions are easy to access and beneficial to our players. Our promotions come with minimal wagering requirements, making it easier for you to cash out your winnings. Moreover, the variety of games we offer ensures that there’s something for every type of player, from classic slot enthusiasts to those who enjoy more modern, feature-packed games.
Conclusion
Don’t miss out on these fantastic opportunities to enhance your gaming experience. Whether you’re looking to enjoy bonus spins, cashback, or bountiful deposit bonuses, we have something for everyone. Join us today, take advantage of these incredible promotions, and start your journey to big wins and endless fun. Happy gaming!
সবচেয়ে জনপ্রিয় খেলা এবং স্বাগত বোনাস
স্বাগত বোনাস এবং অন্যান্য আকর্ষণীয় বোনাসগুলি পেতে এবং সরাসরি ক্রিকেট এক্সচেঞ্জে অংশগ্রহণ করে আপনি এখনি সহযোগিতা করতে পারেন ক্রিকেট এফিলিয়েট প্রোগ্রামের মাধ্যমে। এই প্রোগ্রামের মাধ্যমে আপনি সাধারণ আয় করতে পারেন এবং সাথেই অন্যান্য সুযোগ পাওয়ার অধিকার দিতে পারেন।
এই রোমাঞ্চকর বোনাস গুলি যেগুলি আপনি উপভোগ করতে পারেন এবং আরও অনেক কিছুই আপনাকে আকর্ষণ করতে পারে। প্রথম আমানতে 200% বোনাস পান এবং প্রতিদিন ১০০% বোনাস পেতে অংশগ্রহণ করুন। এছাড়াও, সাপ্তাহিক এফবি শেয়ার করে ১০০ টাকা বোনাস পান।
এই সময়ে ক্রিকেট এক্সচেঞ্জ সরাসরি সরাসরি খেলা এবং জনপ্রিয় খেলা এবং জনপ্রিয় খেলা – ব্যাকারাট, লাইভশো, ফিশিং, এবং স্লট আমাদের নিখুঁতভাবে চেষ্টা করুন। এই সাইটে আপনি 9wicket, crickex affiliate login এবং crickex login সাথে যোগাযোগ করতে পারেন।
তাহলে, আপনি কি অপেক্ষা করছেন? এখনই যোগ দিন এবং সবচেয়ে জনপ্রিয় খেলা এবং স্বাগত বোনাস পান!
Welcome to Cricket Affiliate | Kick off with a smashing Welcome Bonus !
First Deposit Fiesta! | Make your debut at Cricket Exchange with a 200% bonus.
Daily Doubles! | Keep the scoreboard ticking with a 100% daily bonus at 9wicket!
#cricketaffiliate
IPL 2024 Jackpot! | Stand to win ₹50,000 in the mega IPL draw at cricket world!
Social Sharer Rewards! | Post and earn 100 tk weekly through Crickex affiliate login.
https://www.cricket-affiliate.com/
#cricketexchange #9wicket #crickexaffiliatelogin #crickexlogin
crickex login VIP! | Step up as a VIP and enjoy weekly bonuses!
Join the Action! | Log in through crickex bet exciting betting experience at Live Affiliate.
Dive into the game with crickex live—where every play brings spectacular wins !
https://rybalka-v-rossii.ru/ – сайт о рыбалке в России, способах ловли рыб, и выборе правильных снастей.
Found a captivating read that I’d like to recommend to you https://telugusaahityam.com/ упить_?иплом:_Ѕыстро_и_Ћегально
Чистка после пожара в Москве https://prof-uborka-posle-pozhara.ru/
lee bet lee bet
Наш сайт эротических рассказов https://shoptop.org/ поможет тебе отвлечься от повседневной суеты и погрузиться в мир страсти и эмоций. Богатая библиотека секс историй для взрослых пробудит твое воображение и позволит насладиться каждой строкой.
Came across an interesting article, I propose you have a look https://brandnewday.ru/services/istoriya-i-osobennosti-elitnogo-eskorta/
На сайте https://ruuserial.net/ посмотрите интересные, увлекательные, новые, а также полюбившиеся сериалы, которые откроют для вас новые горизонты. В них играют талантливые актеры, которые у всех на устах. Для того чтобы подобрать определенный фильм, необходимо воспользоваться специальным фильтром по жанру: комедия, триллер, драма, криминал, детективы, военные. Также получится сделать подборку и по году выпуска. Регулярно на сайте происходят обновления, чтобы вы посмотрели такое кино, которое хочется.
Найти хорошего детского психолога — задача не из легких, но она крайне важна для гармоничного развития ребенка. Такой специалист поможет справиться с эмоциональными и поведенческими проблемами, которые могут возникать в детстве. В клинике AllegroVision – [url=https://allegrovision.ru/]лазерная стимуляция зрения[/url] .ru работают высококвалифицированные психологи, которые находят индивидуальный подход к каждому ребенку. Они помогают детям развивать уверенность в себе, справляться с тревогами и страхами, а также налаживать отношения со сверстниками и взрослыми.
Хороший детский психолог умеет выслушать ребенка и его родителей, понимает их переживания и помогает найти оптимальные решения. Важно, чтобы сеансы проходили в дружелюбной и доверительной атмосфере, что способствует раскрытию детских чувств и мыслей. В AllegroVision – [url=https://allegrovision.ru/nocity/]коррекция почекра у детей ижевск[/url] все специалисты обладают необходимыми знаниями и опытом, чтобы помочь вашему ребенку стать счастливее и успешнее.
выкуп кредитных авто https://vykup-avtomsk.ru
বাংলাদেশের শীর্ষ অনলাইন ক্যাসিনো প্ল্যাটফর্ম JiliAce〡Jitaace
বাংলাদেশের প্রধান অনলাইন ক্যাসিনো প্ল্যাটফর্ম হিসেবে JiliAce〡Jitaace নিজেকে স্থাপন করেছে, যেখানে নিরাপত্তার সাথে উত্তেজনার সমন্বয় করা হয়েছে। এই প্ল্যাটফর্মটি ব্যবহারকারীদের জন্য ক্রিকেট ক্যাসিনো স্লট গেম, লাইভ ক্যাসিনো, লটারি, স্পোর্টসবুক, স্পোর্টস এক্সচেঞ্জ এবং ই-স্পোর্টের বিস্তৃত নির্বাচন অফার করে। JiliAce〡Jitaace ২৪-ঘণ্টা বন্ধুত্বপূর্ণ লাইভ গ্রাহক সহায়তা প্রদান করে, যেকোন প্রশ্ন দ্রুত মোকাবেলা করা এবং সমাধান করা হয়েছে তা নিশ্চিত করতে। এছাড়াও, প্ল্যাটফর্মটি বিভিন্ন নিরাপদ এবং সহজ অর্থপ্রদানের পদ্ধতি অফার করে, যা ব্যবহারকারীদের জন্য লেনদেন সহজ এবং নির্ভরযোগ্য করে তোলে।
এই প্ল্যাটফর্মটি ব্যবহারকারীদের জন্য ইভিও মানি হুইল, জিলি স্লট, স্প্রাইব ক্র্যাশ এবং আরও অনেক সুপরিচিত গেমগুলির একটি পরিসর নিয়ে আসে। আপনি ক্র্যাশ গেম, স্লট, ফিশিং, লাইভ গেমস, অথবা স্পোর্টস বেটিং পছন্দ করুন না কেন, JiliAce〡Jitaace-এর কাছে সবকিছুই রয়েছে। এই প্ল্যাটফর্মটি প্রতিটি ব্যবহারকারীর জন্য বিভিন্ন ধরণের গেমের একটি অসাধারণ অভিজ্ঞতা প্রদান করে, যা তাদের জয়কে আরও উত্তেজনাপূর্ণ করে তোলে।
JiliAce〡Jitaace-এর সাথে যুক্ত হন এবং বাংলাদেশের সবচেয়ে জনপ্রিয় ক্যাসিনো অ্যাপে উত্তেজনাপূর্ণ পুরস্কার জিতে নিন! আপনার বিজয়ের জন্য অসাধারণ পুরস্কার অপেক্ষা করছে, তাই আজই যোগ দিন এবং অভিজ্ঞতা নিন বাংলাদেশের শ্রেষ্ঠ অনলাইন ক্যাসিনো প্ল্যাটফর্ম JiliAce〡Jitaace-এর।
Jiliacet casino
Jiliacet casino |
Warm welcome! | Get a 200% Welcome Bonus when you log in jiliace casino.
IPL Tournament! | Join the IPL fever with Jita Bet. Win big prizes!
Epic JILI Tournaments! | Go head-to-head in the Jita bet Super Tournament
#jiliacecasino
Cashback on Deposits! | Get cash back on every deposit. At Jili ace casino
Uninterrupted bonuses at JITAACE! | Stay logged in Jiliace login!
https://www.jiliace-casino.online/bn
#Jiliacecasino # Jiliacelogin#Jiliacelogin # Jitabet
Slot Feast! | Spin to win 100% up to 20,000 ৳. at Jili ace login!
Big Fishing Bonus! | Get a 100% bonus up to ৳20,000 in the Fishing Game.
JiliAce Casino’s top choice for great bonuses. login, play, and win big today!
выкуп авто продать авто автовыкуп машин
лечение алкоголизма в домашних условиях https://narcolog-expert.ru/
Установка крыши на балконы спб specbalkon.ru
Компания СПЕЦ Балкон оказывает услуги по замене остекления, утеплению и отделке балконов в Спб. Мы концентрируется конкретно на балконах, что даёт нам сосредоточиться на качестве и скорости работы. При заказе на сайте specbalkon.ru остекления и отделки «под ключ» мы дарим скидку в десять %. Прямо сейчас переходите на наш интернет портал и оформляйте заявку.
Если Вам нужно [url=https://specbalkon.ru/zamena-ostekleniya]холодное остекление на теплое цена[/url] прямо сегодня, то мы вам обязательно окажем помощь. Услуга по смене холодного фасадного остекления по теплое сейчас весьма востребована. При покупке жилья в новом доме очень часто застройщик ставит на балкон холодное, не очень качественное остекление, которое лучше поменять сразу же после покупки. Теплое остекление лоджии имеет множество преимуществ: окна не замерзают даже в самые холодные зимы, на балконе можно сделать отдельное помещение, цветы будут стоять при комфортной температуре, на балконе можно сделать места для хранения и вещи не деформируются. Поэтому предлагаем Вам замену остекления сделать как можно скорее и по выигрышной цене.
Относительно [url=https://specbalkon.ru/zamena-ostekleniya]фасадное остекление балкона спб[/url] на выгодных условиях, приходите к нам. Контактный номер телефона +7(812)240-94-42 или закажите обратный звонок на указанном сайте. Мы осуществляем монтаж в точно установленный срок, часто в течение 1-3 дней. Даётся гарантия на изделия в среднем 5 лет, на работу — 3 года. Оплата осуществляется по факту исполненных работ, предоплата не потребуется.
Компания specbalkon.ru находится по адресу: Санкт-Петербург, ул. Оптиков, д. 4. Режим работы с пн по вс с 10:00 до 19:00. Также мы изготавливаем крыши на балконы с установкой, внешнюю отделку парапета, внутреннюю отделку балкона по любому дизайну. Звоните, приходите, будем счастливы с Вами сотрудничать.
как напугать алкоголика чтобы бросил пить https://medutox.ru/
лечение алкоголизма гипнозом https://detoxme.kz/
https://formomebel.ru/krovati
nanosluchatka mikrosluchatko
На сайте https://polygrav.ru вы сможете заказать такую услугу, как производство бейджей, табличек, а также номерков, шильдиков в огромном ассортименте. Только в этой компании вы отыщите огромное количество наиболее выгодных, интересных предложений. Производство является высокотехнологичным, а потому есть возможность заказать товары в любом количестве. Также вы сможете приобрести таблички на дверь, номерки для гардероба. Вся продукция производится из высокотехнологичных материалов, которые не испортят внешний вид.
купить микронаушники https://jasdam.cz/
https://rybalka-v-rossii.ru – сайт о рыбалке в России, способах ловли рыб, и выборе правильных снастей.к
купить зимние грузовые шины
al-club.ru либет казино
купить флешки оптом https://meflash.ru/
операция по увеличению полового члена
https://operacziya-uvelichenie-chlena.ru/
консультация бухгалтера спб
Portál szakemberek keresőjének https://szakiweb.hu/ -Látogass el az oldalra és találsz egy mesterembert építkezésre, javításra, ház körüli segítségre. Nagyon könnyű mestert vagy szakembert találni az országban bárhol. Vegye igénybe az oldalon található szakemberek szolgáltatásait.
На сайте https://aakkb.com.ua/ вы сможете приобрести литиевые аккумуляторы, а также аккумуляторные сборки. Все конструкции отличаются своей надежностью, безупречным качеством, а также идеальным исполнением. Все модели от проверенных, лучших брендов, которые работают на совесть. Перед вами огромное количество моделей, что позволит подобрать решение на свое устройство. А если затрудняетесь с выбором, то вас всегда поддержит высококлассный специалист. Он даст ценные советы, важные рекомендации.
https://proauto.kyiv.ua здесь вы найдете обзоры и тест-драйвы автомобилей, свежие новости автопрома, обширный автокаталог с характеристиками и ценами, полезные советы по уходу и ремонту, а также активное сообщество автолюбителей. Присоединяйтесь к нам и оставайтесь в курсе всех событий в мире автомобилей!
Are you looking for reliable and fast proxies? https://fineproxy.org/account/aff.php?aff=29 It offers a wide range of proxy servers with excellent speed and reliability. Perfect for surfing, scraping and more. Start right now with this link: FineProxy.org . Excellent customer service and a variety of tariff plans!
https://autoblog.kyiv.ua путеводитель в мире автомобилей. Обзоры и тест-драйвы, актуальные новости, автокаталог, советы по уходу и ремонту, а также общение с автолюбителями. Всё, что нужно для выбора и эксплуатации авто, вы найдете у нас.
Euro
sunmory33
Understanding the Online Gambling Landscape in the Philippines
The world of online gambling has been rapidly evolving, with the Philippines emerging as a dynamic hub for players seeking thrilling gaming experiences. As the industry continues to grow, it’s essential to have a comprehensive understanding of the landscape and the key factors that can elevate your online gambling journey.
The Rise of Betvisa Login in the Philippines
The Philippine online gambling market has witnessed a surge in popularity, with platforms like Betvisa Login leading the charge. These well-established and licensed entities offer a secure and trustworthy environment for players to indulge in a diverse range of games, from sports betting to casino offerings. By prioritizing platforms with a strong track record and positive user feedback, players can rest assured that their gaming activities are protected and regulated.
Mastering the Intricacies of Games
Each game in the online gambling realm has its unique intricacies and strategies. Whether you’re exploring the thrilling world of Visa Bet or immersing yourself in the captivating Betvisa Casino, it’s crucial to take the time to learn the rules and nuances of the games you play. Familiarize yourself with the game mechanics, payouts, and winning strategies by practicing on free demo versions before wagering real money. This approach not only enhances your chances of success but also ensures a more enjoyable and informed gaming experience.
Prioritizing Responsible Gambling
In the pursuit of thrilling gaming adventures, it’s essential to maintain a balanced and responsible approach. Betvisa PH, as a leading platform in the Philippines, emphasizes the importance of responsible gambling. Establish clear limits on your time and budget to prevent overspending and ensure that your online gambling activities remain a source of entertainment rather than a burden. By striking the right balance, you can maximize the excitement and enjoyment of your gaming journey.
Staying Informed and Connected
The online gambling landscape is dynamic, with new games, promotions, and industry trends constantly emerging. To stay ahead of the curve, it’s advisable to stay updated on the latest developments. Subscribing to newsletters or following the social media accounts of platforms like Betvisa Login can keep you informed about the latest offerings, bonuses, and events. Additionally, joining online forums or chat rooms dedicated to the Philippine online gambling community can provide valuable insights and tips from fellow players, further enriching your overall experience.
Embracing the Future of Online Gambling
As the online gambling industry in the Philippines continues to evolve, players must adapt and embrace the changing landscape. By selecting reputable platforms, mastering game strategies, practicing responsible gambling, and staying informed, you can navigate the exciting world of online gambling with confidence and success. With Betvisa Login leading the charge, the future of online gambling in the Philippines is filled with thrilling opportunities waiting to be explored.
Betvisa Bet | Step into the Arena with Betvisa!
Spin to Win Daily at Betvisa PH! | Take a whirl and bag ?8,888 in big rewards.
Valentine’s 143% Love Boost at Visa Bet! | Celebrate romance and rewards !
Deposit Bonus Magic! | Deposit 50 and get an 88 bonus instantly at Betvisa Casino.
#betvisa
Free Cash & More Spins! | Sign up betvisa login,grab 500 free cash plus 5 free spins.
Sign-Up Fortune | Join through betvisa app for a free ?500 and fabulous ?8,888.
https://www.betvisa-bet.com/tl
#visabet #betvisalogin #betvisacasino # betvisaph
Double Your Play at betvisa com! | Deposit 1,000 and get a whopping 2,000 free
100% Cock Fight Welcome at Visa Bet! | Plunge into the exciting world .Bet and win!
Jump into Betvisa for exciting games, stunning bonuses, and endless winnings!
https://autoclub.kyiv.ua узнайте все о новых моделях, читайте обзоры и тест-драйвы, получайте советы по уходу за авто и ремонтам. Наш автокаталог и активное сообщество автолюбителей помогут вам быть в курсе последних тенденций.
https://ktm.org.ua/ у нас вы найдете свежие новости, аналитические статьи, эксклюзивные интервью и мнения экспертов. Будьте в курсе событий и тенденций, следите за развитием ситуации в реальном времени. Присоединяйтесь к нашему сообществу читателей!
https://mostmedia.com.ua мы источник актуальных новостей, аналитики и мнений. Получайте самую свежую информацию, читайте эксклюзивные интервью и экспертные статьи. Оставайтесь в курсе мировых событий и тенденций вместе с нами. Присоединяйтесь к нашему информационному сообществу!
Founded in Texas in 2002, Del Mar Energy quickly transformed into one of the leading players in the energy market, oil and gas extraction, road construction
Фасадное остекление балкона в СПб specbalkon.ru
Если Вам требуется [url=https://specbalkon.ru/zamena-ostekleniya]фасадное остекление санкт петербург[/url] прямо сейчас, то мы вам обязательно поможем. Услуга по замене холодного фасадного остекления по теплое сейчас весьма востребована. При покупке жилья в новом доме очень часто застройщик ставит на балкон холодное, не очень хорошее остекление, которое лучше менять сразу же после приобретения. Теплое остекление лоджии имеет множество плюсов: окна не замерзают даже в самые холодные зимы, на балконе возможно сделать отдельное пространство, цветы будут стоять при комфортной температуре, на балконе можно сделать места для хранения и вещи не испортятся. Поэтому предлагаем Вам замену остекления сделать как можно скорее и по лучшей цене.
https://kursovyemetrologiya.ru
PRO88: Situs Game Online Deposit Pulsa Terbaik di Indonesia
PRO88 adalah situs game online deposit pulsa terbaik tahun 2020 di Indonesia. Kami menyediakan berbagai macam game online terbaik dan terlengkap yang bisa Anda mainkan di situs game online kami. Hanya dengan mendaftar satu ID, Anda bisa memainkan seluruh permainan yang tersedia di PRO88.
Keunggulan PRO88
PRO88 juga merupakan situs agen game online berlisensi resmi dari PAGCOR (Philippine Amusement Gaming Corporation), yang berarti situs ini sangat aman. Kami didukung dengan server hosting yang cepat dan sistem keamanan dengan metode enkripsi termutakhir di dunia untuk menjaga keamanan database Anda. Selain itu, tampilan situs kami yang sangat modern membuat Anda nyaman mengakses situs kami.
Berbagai Macam Game Online
Kami menyediakan berbagai macam game online terbaik yang bisa Anda mainkan kapan saja dan di mana saja. Dengan satu ID, Anda bisa menikmati semua permainan yang tersedia, mulai dari permainan kartu, slot, hingga taruhan olahraga. PRO88 memastikan semua game yang kami sediakan adalah yang terbaik dan terlengkap di Indonesia.
Keamanan dan Kenyamanan
Kami memahami betapa pentingnya keamanan dan kenyamanan bagi para pemain. Oleh karena itu, PRO88 menggunakan server hosting yang cepat dan sistem keamanan dengan metode enkripsi termutakhir di dunia. Ini memastikan bahwa data pribadi dan transaksi Anda selalu aman bersama kami. Tampilan situs yang modern juga dirancang untuk memberikan pengalaman bermain yang nyaman dan menyenangkan.
Kesimpulan
PRO88 adalah pilihan terbaik untuk Anda yang mencari situs game online deposit pulsa yang aman dan terpercaya. Dengan berbagai macam game online terbaik dan terlengkap, serta dukungan keamanan yang canggih, PRO88 siap memberikan pengalaman bermain game yang tak terlupakan. Daftar sekarang juga di PRO88 dan nikmati semua permainan yang tersedia hanya dengan satu ID.
Kunjungi kami di PRO88 dan mulailah petualangan game online Anda bersama kami!
https://formomebel.ru/stoliki/metallicheskie
Категория врача получить на maps-edu.ru
Дистанционное обучение сегодня с каждым днем становится только востребованнее. Это комфортно, недолго и выгодно, при том, что информативность обучения не теряется и Вы получаете официальные документы после завершения. Академия «МАПС» выложила информацию о своих программах на веб портале maps-edu.ru и окажет помощь с выбором программы, нужной определенно Вам.
Относительно [url=https://maps-edu.ru/nabor-ballov-nmo]купить баллы нмо[/url] – Вы на правильном пути. Направления, по которым мы можем обучить: строительство, агрономия и сельское хозяйство, педагогика, физическая культура и спорт, пожарная безопасность, закупки, экономика и финансы, документоведение и делопроизводство, менеджмент, антитеррористическая защищенность и многие другие. С учениками заключаем договор и работаем по его плану. Имеем все нужные лицензии на обучающую деятельность, а по итогу все документы отправляются в ФИС ФРДО. У нас уже много выпускников прошли обучение и написали отзывы, прочтите их прямо сейчас на указанном интернет портале.
Особенно хочется отметить набор услуг для медицинских работников. Это аккредитация, профессиональная переподготовка, получение медицинской категории, обучение младшего медперсонала и другие. Данная категория весьма популярна, потому как медицина в нашей стране развивается ежедневно. Чтобы быть в теме всех новых способов лечения, лекарственных препаратов, аппаратов, врачам нужно регулярно проходить повышение квалификации. А способ удаленно подходит всем без исключения. Удобно и недорого в наше непростое время.
По теме [url=https://maps-edu.ru/catalog/logopediia?type=professionalnaya-perepodgotovka]где пройти курсы переподготовки на логопеда[/url] онлайн, мы Вам обязательно окажем помощь. Звоните по контактному телефону 8(800)777-06-74 и задавайте все возникшие вопросы. Звонок по России бесплатный. Регионально находимся по адресу: г. Иркутск, ул. Степана Разина, д. 6. На сайте maps-edu.ru вы также можете обратиться в службу помощи и Вас проконсультирует наш менеджер.
Друзья, вы не поверите, но эти телеграм-каналы сейчас на устах у всех! Кажется, что все говорят о [url=https://t.me/s/kino_film_20221]новинки кино 2024[/url] – самые горячие премьеры и захватывающие обсуждения только здесь. Для тех, кто хочет заработать в интернете, канал [url=https://t.me/s/zarabotak_sam]заработок в интернете[/url] стал настоящим открытием – только полезная и проверенная информация. Если вас интересует саморазвитие, то [url=https://t.me/s/razvisam]саморазвитие с чего начать[/url] – лучший выбор. Актуальные и полезные советы каждый день! В финансовом мире все говорят о [url=https://t.me/s/new_mfo]новые МФО 2024[/url], а для срочных займов все выбирают [url=https://t.me/s/MFO_dayt_vsem]займы которые дают всем[/url]. Подписывайтесь, чтобы быть в тренде!
На сайте https://aakkb.com.ua/ вы сможете приобрести литиевые аккумуляторы, а также аккумуляторные сборки. Все конструкции отличаются своей надежностью, безупречным качеством, а также идеальным исполнением. Все модели от проверенных, лучших брендов, которые работают на совесть. Перед вами огромное количество моделей, что позволит подобрать решение на свое устройство. А если затрудняетесь с выбором, то вас всегда поддержит высококлассный специалист. Он даст ценные советы, важные рекомендации.
купить самую реалистичную секс куклу https://24sexy-dolls.ru
MIGRANT SUPPORT предлагает помощь в получении номера PESEL и гарантирует разумные цены. Профессиональные специалисты компании, прилагают максимум усилий для выполнения различных задач. https://migrant.support/poluchieniie_nomiera_pesel – сайт, где узнаете, для чего нужен песель и как, сделать его удаленно. Мы быстро и качественно оказываем помощь, а также предлагаем абсолютно бесплатно консультацию. К своим клиентам относимся лояльно, и делаем все, чтобы их ожидания были оправданы. Обращайтесь именно к нам и не пожалеете о своем выборе!
https://fraza.kyiv.ua/ вы найдете последние новости, глубокие аналитические материалы, интервью с влиятельными личностями и экспертные мнения. Следите за важными событиями и трендами в реальном времени. Присоединяйтесь к нашему сообществу и будьте информированы!
На сайте https://setki.moscow вы сможете оформить заявку для того, чтобы заказать москитную сетку. Конструкции производятся из качественных, надежных материалов и всего за день. Монтажные работы проводятся в любой, наиболее комфортный для вас день. На работы, конструкции даются гарантии 12 месяцев. Рассчитать итоговую цену вы сможете прямо сейчас. Установлена привлекательная стоимость за счет собственного производства. Работа происходит не только по Москве, но и области. Предприятие специализируется на производстве, реализации, а также установке москитных сеток.
На сайте https://ax-card.ru/ закажите пластиковые карты для различных сфер деятельности. Их создают на инновационном, высокотехнологичном оборудовании компетентные специалисты с огромным опытом. Дополнительно они разработают макет, а при оказании услуги используют уникальные и инновационные технологии. Именно поэтому вы получите ожидаемый и прогнозируемый результат. Если вы заинтересовались услугой, то специально для вас может быть сделана пробная карта. Это абсолютно бесплатно.
Stumbled upon a unique article, I suggest you take a look http://talkrealty.ru/individualki-irkutska-vashi-samyie-smelyie-fantazii
Консультация по оптимизации продвижению.
Информация о том как работать с низкочастотными запросами и как их выбирать
Подход по действиям в конкурентоспособной нише.
Имею постоянных клиентов работаю с 3 организациями, есть что поделиться.
Изучите мой профиль, на 31 мая 2024г
общий объём успешных проектов 2181 только в этом профиле.
Консультация проходит в устной форме, никаких скринов и отчетов.
Продолжительность консультации указано 2 ч, но по реально всегда на связи без твердой привязки ко времени.
Как управлять с программным обеспечением это уже другая история, консультация по работе с софтом договариваемся отдельно в другом кворке, определяем что необходимо при разговоре.
Всё спокойно на без напряжения не спеша
To get started, the seller needs:
Мне нужны данные от телеграмм чата для связи.
общение только в устной форме, общаться письменно не хватает времени.
субботы и воскресенья выходные
https://bestwoman.kyiv.ua узнайте всё о моде, красоте, здоровье и личностном росте. Читайте вдохновляющие истории, экспертные советы и актуальные новости. Присоединяйтесь к нашему сообществу женщин, живущих яркой и насыщенной жизнью!
На сайте https://cryptocodes.kz/ ознакомьтесь с интересной и полезной информацией о криптовалюте. Здесь находится все, что вам необходимо знать о криптографии. На сайте вы найдете и промокоды, другую важную и полезную информацию, которая обязательно вам пригодится. Подобные бонусы помогут вам значительно сэкономить, чтобы вы смогли получить больше приятных эмоций. Также вы получите и ответы на многочисленные вопросы, которые помогут вам правильно обращаться с криптовалютой. Регулярно на портале появляется новая, свежая информация.
На сайте https://t.me/mvavada почитайте интересную, увлекательную и свежую информацию относительно проекта «VAVADA», который стал одним из самых популярных в Интернете. У вас появляется удивительная возможность ознакомиться с материалами, которые будут нужны для того, чтобы воспользоваться всеми преимуществами онлайн-площадки. На этом канале, в первую очередь, публикуются ценные и важные новости, промокоды, освещаются важные события. Публикуются только честные, правдивые материалы. Заходить сюда можно ежедневно и с любого устройства, в том числе, смартфона.
Грузовики HOWO и Sinotruk в наличии и под заказ на https://partnerauto.ru/ от компании ООО «Новая высота» – это возможность выгодно купить китайские грузовики с доставкой в любую точку РФ. Доступные цены и удобный сервис. Подробнее на сайте.
сериалы в хорошем качестве
Курсы переподготовки для педагогов maps-edu.ru
Относительно [url=https://maps-edu.ru/specialties/courses/socialnaia-rabota-obespecenie-realizacii-socialnyx-uslug-i-mer-socialnoi-podderzki-naseleniia-1]курсы социального педагога[/url] – Вы на правильном пути. Направления, по которым мы обучаем: охрана труда, медицина, бухгалтерское дело, дефектология, юриспруденция и право, социальная работа, экономика и финансы, документоведение и делопроизводство, нефтяная и газовая промышленность, метрология и стандартизация и многие другие.
На сайте https://xn--57-6kchpq9cd0i.xn--p1ai/ закажите натяжные потолки высокого качества и выполненные из современных, высокотехнологичных материалов, которые не утратят своего внешнего вида долгое время. На всю продукцию установлены привлекательные, доступные расценки, а работы выполняются точно в срок и квалифицированными мастерами. Все услуги оказываются «под ключ», предоставляются гарантии. Предоставляется скидка 10%. Монтажные работы выполняются всего за пару часов. На консультации вам помогут с выбором и подскажут, какая конструкция подходит именно вам.
На сайте https://oboinastene.ru/ почитайте информацию об обоях. Вы узнаете об их видах, особенностях, преимуществах, использовании в интерьере. Кроме того, есть информация о том, как их правильно клеить, чтобы они красиво выглядели в течение долгого времени. Есть данные о ремонте кровли, о том, как правильно его выполнять. Все обои различаются между собой не только цветовой гаммой, но и фактурой, а также типом поверхности, текстурой и другими особенностями. Есть информация и об автоматических выключателях ДКС.
Вас интересует прописка в Польше, г. Варшава? MIGRANT SUPPORT вам в этом поможет. Компания предлагает высококачественные услуги. Гарантирует лояльные цены и персональный подход к каждому клиенту. У вас есть возможность получить квалифицированную консультационную поддержку по необходимым вопросам. https://vpolshe.com/propiska-v-polshe-g-varshava-dogovor-arendy-zhilya-varshava/ – сайт, где можно узнать, зачем нужна прописка (meldunek). Оставьте заявку и мы свяжемся с вами в ближайшее время. С удовольствием поможем с договором аренды жилья.
https://prowoman.kyiv.ua на нашем сайте вы найдете полезные советы по моде, красоте, здоровью и отношениям. Читайте вдохновляющие статьи, участвуйте в обсуждениях и обменивайтесь идеями. Присоединяйтесь к нашему сообществу современных женщин!
Зайдите на сайт https://came.name/ где вы сможете ознакомиться с широким ассортиментом оборудования CAME (итальянский производитель), таким как автоматика для ворот, шлагбаумы, парковочные системы, турникеты и многим другим. Узнайте больше информации о продукции на сайте, а также посмотрите прайс листы с выгодными ценами.
Зайдите на сайт https://deep-well.ru/ где вы сможете заказать такие полезные услуги как чистка и ремонт колодцев в Москве. Компания Глубокий колодец работает на рынке с 2003 года и оказывает профессиональные услуги по чистке, ремонту, углублению колодцев, а наши мастера знают о них все. Стоимость услуг доступна каждому. Подробнее на сайте.
http://wiki.die-karte-bitte.de/index.php/Benutzer_Diskussion:LukeZ63948917
buy cheap tiktok live views Buy TikTok Live Views
https://superwoman.kyiv.ua вы на нашем надежном гиде в мире женской красоты и стиля жизни! У нас вы найдете актуальные статьи о моде, красоте, здоровье, а также советы по саморазвитию и карьерному росту. Присоединяйтесь к нам и обретайте новые знания и вдохновение каждый день!
Посетите сайт https://seovgeo.ru/ где вы сможете заказать продвижение на яндекс картах, продвижение компании в яндекс картах, продвижение организации на яндекс картах, сео продвижение на яндекс картах, продвижение через яндекс карты и многие другие услуги с индивидуальным подходом от профессионалов по выгодным ценам. Подробнее на сайте.
bocor88
решения задач на заказ https://resheniezadachmatematika.ru/
На сайте https://us-canad.com/ изучите карты автомобильных дорог Канады, США. Только здесь вас ожидает самый подробный, детальный атлас всех дорог, которые находятся в Северной Америке. Для каждого посетителя сайта доступны крупномасштабные, полноразмерные карты, где учитываются и маршруты США, а также расстояние в милях. Имеется атлас с границами округов. Представлена карта всех штатов вместе с автомагистралями. Перед вами подробные цветные карты всех штатов США, где присутствуют памятники, национальные парки и многое другое.
На сайте https://dolinakamnej.ru приобретите минералы, натуральные камни, а также интересные и необычные изделия, которые понравятся даже истинному эстету. Доставка осуществляется в любую точку мира, России. Компания отличается собственным производством, а потому установила привлекательные и доступные цены. Здесь же есть возможность воспользоваться и такими популярными, нужными услугами, как полировка, распиловка на заказ. Все услуги оказываются качественно, в соответствии с самыми высокими требованиями.
курсовые на заказ https://kursovyematematika.ru
Visit https://quasa.io/ and meet Quasa, which connects people who need home or remote services with pre-vetted, independent professionals who can help with home services or remote work and errands around the city: household services, photo and video, editing, installation, setup, training, courses, preparation, remote work and more.
Search by Image on Amazon https://chromewebstore.google.com/detail/search-by-image-on-amazon/fmiialomjacaogdjjkgicepphmekndli uses advanced technology to provide users with a unique functionality. By analyzing images, the extension is able to search for relevant products. To use this feature, users can conveniently right-click on any image in their browser and select the “Search on Amazon” option.
Ассоциация Безопасности Трейдинга https://trading-association.org/ это помощь в возврате денег от брокера. Зайдите на сайт, ознакомьтесь с ассоциацией, способами решения сложностей с проблемными брокерами или получите консультацию. Подробнее на сайте.
Came across an interesting article, I propose you have a look https://www.podchaser.com/podcasts/betpromocod-5751934
заказать курсовую онлайн https://kursovyebankovskoe.ru/
Сайт https://zhenskiy.kyiv.ua – це онлайн-ресурс, який присвячений жіночим темам та інтересам. Тут зібрана інформація про моду, красу, здоров’я, відносини, кулінарію та багато іншого, що може бути корисним та цікавим для сучасних жінок.
Обучение дистанционно maps-edu.ru
Насчёт [url=https://maps-edu.ru/specialties/courses/socialnaia-rabota-obespecenie-realizacii-socialnyx-uslug-i-mer-socialnoi-podderzki-naseleniia-1]социальная педагогика переподготовка[/url] – Вы на точном пути. Направления, по которым мы можем обучить: строительство, агрономия и сельское хозяйство, бухгалтерское дело, дефектология, пожарная безопасность, закупки, экономика и финансы, документоведение и делопроизводство, менеджмент, метрология и стандартизация и многие другие.
На сайте https://nichego-nebolit.ru/ ознакомьтесь с любопытной, интересной информацией, рекомендациями, которые касаются того, как подобрать каре, каковы преимущества и нюансы данной стрижки. Имеются данные о КТ легких и о том, когда лучше всего проводить процедуру и в каких случаях. Есть информация и о системах для нагревания табака. Даны рекомендации о том, как правильно выбрать брус. Есть любопытная статья об озонотерапии и том, как она может вам помочь в улучшении состояния здоровья.
Доставка цветов в Саратове https://flowers64.ru/ это отличная возможность заказать различные цветы, букеты, композиции, подарки, не выходя из дома.
приложение бк leon
На сайте https://rating-smart.ru/ узнайте стоимость такой популярной и важной услуги, как написание и размещение отзывов с гарантией. Специалисты сервиса разместят отзывы, после чего поднимут рейтинг вашего предприятия на всех возможных площадках, а также порталах-отзовиках. Работа осуществляется по всем городам России. На все услуги предоставляются гарантии. Все отзывы публикуются исключительно с настоящих аккаунтов. Отзывы повышают лояльность клиентов, доверие к вашему продукту или услугам.
Не знаете, где взять деньги срочно? Наш канал [url=https://t.me/s/zaim_srochno_bez_otkaza_na_kartu]срочные займы[/url] предлагает самые надежные и проверенные МФО. Займы на карту без отказа, быстро и удобно. Подписывайтесь и будьте в курсе всех выгодных предложений!
Came across a unique article – it’s worth your attention https://gamerplayz2k.mn.co/posts/58244720
заказать курсовую работу https://kupit-kursovuyu-rabotu.ru/ с гарантией и антиплагиатом
На сайте https://pacific-map.com/index.html представлены карты с городами, штатами. Перед вами самая подробная карта дорог, которые имеются в США. Крупномасштабные карты содержат самое подробное описание, координаты, а потому вы можете ориентироваться на данную информацию. Это огромная карта со всеми подробностями и важной информацией. Есть карта со штатами, а также Атлантического, Тихоокеанского Побережья. Открыть карту получится с любого устройства и где бы вы ни находились. Все детали отлично видны.
Компания «СХТ» предлагает вам приобрести высокоточное измерительное оборудование. Мы даем гарантию на выгодные цены и отменное качество нашей продукции. Наши специалисты обладают высокой квалификацией и большим опытом работы. Они с удовольствием проконсультируют вас по всем необходимым вопросам. https://cxt.su – сайт, где у вас есть возможность посмотреть детальнее наши услуги и информацию. Здесь вы найдете видео-презентацию и фотогалерею. На все заявки мы быстро реагируем. Обращайтесь именно к нам!
Консультация по стратегии продвижения сайтов продвижению.
Информация о том как взаимодействовать с низкочастотными запросами ключевыми словами и как их подбирать
Подход по деятельности в конкурентоспособной нише.
Имею постоянных клиентов работаю с тремя фирмами, есть что поделиться.
Ознакомьтесь мой досье, на 31 мая 2024г
число завершённых задач 2181 только в этом профиле.
Консультация только в устной форме, никаких скринов и отчётов.
Длительность консультации указано 2 часа, и сути всегда на связи без жёсткой фиксации времени.
Как управлять с ПО это уже отдельная история, консультация по работе с софтом обсуждаем отдельно в отдельном разделе, выясняем что требуется при общении.
Всё спокойно на расслабоне не в спешке
To get started, the seller needs:
Мне нужны контакты от телеграм каналов для коммуникации.
общение только в устной форме, переписываться нету времени.
субботы и воскресенья выходной
Зайдите на сайт https://clean-ru.com/ и вы сможете заказать такие услуги как: дезинфекция, дератизация, дезодорация, дезинсекция от профессионалов своего дела, в Москве. Мы специализируемся на предоставлении широкого спектра услуг по отличным ценам, а наши комплексные позволят обеспечить уничтожение различных видов вредителей и бактерий. Узнайте больше на сайте.
На сайте https://vpolshe.com/kriptolitsenziya-v-polshe/ оформите заявку для того, чтобы воспользоваться такой нужной и важной услугой, как получение криптолицензии в Польше. В компании работают компетентные, лучшие сотрудники, которые отличаются огромным опытом. Они дадут вам консультацию, расскажут обо всех достоинствах процедуры, а также о том, какие документы рекомендуется подготовить. Компания уже длительное время оказывает услуги в этой области. Стоимость такой услуги – 10 000 евро. Ее проводят в несколько этапов.
Компания https://primeinterior.ru/ занимается поставками натурального мрамора, гранита, травертина, оникса и кварцита со всего мира для премиальных интерьеров. Имеем собственное производство во Владивостоке и команду специалистов для монтажа. Готовы к крупным заказам любой сложности со всей России, есть опыт работы с отелями, ресторанами, ночными клубами. Создаём неповторимые интерьеры из природного камня, высокое качество работы и материалов.
матрас или матрац купить [url=https://www.kupit-matras111.ru]https://www.kupit-matras111.ru[/url] .
Больше интересной информации о строительстве и ремонте можно прочитать на сайте https://stroyka-gid.ru. Только самые популярные статьи и обзоры процесса ремонта помещений и строительства зданий.
Больше интересной информации о строительстве и ремонте можно прочитать на сайте https://stroyka-gid.ru. Только самые популярные статьи и обзоры процесса ремонта помещений и строительства зданий.
Ищите глиняную посуду ручной работы? Зайдите на сайт https://gzhelgonchar.ru/ и вы найдете широкий ассортимент глиняной посуды от производителя ручной работы. Гжельская гончарня – это широкий ассортимент посуды из глины, которую можно купить с доставкой по РФ и СНГ. Подробнее на сайте.
На сайте https://sales-sport.ru/ почитайте интересные и содержательные материалы на тему моды, красоты, здоровья. Перед вами самые интересные, свежие публикации, которые точно вам понравятся. Вы узнаете о том, как правильно подобрать женские сапоги, короткое свадебное платье, а также брюки для подростка. Имеются материалы о том, как подобрать солнцезащитные очки, про проектирование, а также монтаж инженерных коммуникаций. Также есть данные о том, правильно выровнять бетонный пол, как подобрать стоматологическую клинику.
Компания «Новые технологии и материалы» занимается изготовлением комплектующих для промышленности радиотехнической. Предлагаем вам по выгодной стоимости купить метизы и крепеж. Строго следим за качеством продукции и поставляем ее в указанные сроки. Готовы предоставить профессиональную консультацию и оказать помощь в оформлении товара. https://ntmsp.ru/ – сайт, где у вас есть возможность уже сегодня посмотреть акции. У нас трудятся грамотные и надежные люди. Позвоните нашим специалистам, и они ответят на все необходимые вопросы.
Discovered an article that will definitely interest you – don’t miss the chance to familiarize yourself https://vipka.mybb.ru/viewtopic.php?id=2814#p4904
Crypto Pump Signals for Binance https://cryptopumpsignalsbinance.com/ is a unique project that provides traders and investors with valuable insights into upcoming cryptocurrency pumps. Through its Telegram channel and AI capabilities, this project offers new opportunities for generating massive profits in the short-term.
targeted youtube views buy online contest votes
На сайте https://genshin-news.ru/ вы отыщите новости, гайды популярных игр. Также здесь представлена различная интересная информация об играх, новости. Все публикации составлены экспертами, которые хорошо понимают в этой теме. Есть раздел с такой информацией, с которой вы сможете ознакомиться в данный момент. К примеру, есть баннеры 2020-2024, анонсы, даты выхода, а также сюжет различных игр. Обновления информации происходят каждый день. Поэтому и вы следите за ожидаемыми новинками, с которыми вам необходимо ознакомиться первым.
Czech health insurance for foreigners in Prague https://vaclavak48.cz/ is comprehensive health insurance for foreigners in the Czech Republic at the best prices. Центр Страхования Иностранцев в Праге, Чехия – это Медицинское страхование иностранцев в Чехии, а также Комплексные медицинские страховки для иностранных студентов в Праге и Чехии по лучшим ценам от страховых компаний. Все услуги в одном месте. Узнайте подробнее на сайте.
https://www.instagram.com/asiapsiholog_family/
На сайте https://vezuviy.shop/ приобретите надежные, функциональные печи. Только здесь представлено фирменное оборудование, а также дымоходы, на которые предоставляются гарантии. Для сауны, бани, а также коттеджей вы сможете с легкостью подобрать один из предложенных вариантов. Важным преимуществом компании является то, что доставка предоставляется абсолютно бесплатно, а банный камень дают в подарок. На все аксессуары действует скидка 10%. Дверь для бани вы сможете получить в качестве приятного презента.
vavada рабочее
https://kristall-klining.ru/
На сайте https://ruskiseerial.net/ представлены самые новые, интересные фильмы, сериалы, которые определенно заслуживают вашего внимания. Есть и ожидаемые премьеры, которые хочется посмотреть всем киноманам. Все фильмы в отличном качестве, с хорошей озвучкой. Просматривать можно на любом устройстве, включая планшет, смартфон. Для подбора определенного варианта воспользуйтесь специальным фильтром или поиском по жанру. Здесь есть как драмы, так и мелодрамы, комедии, триллеры. Опубликованы и совсем новые фильмы, вышедшие в этом году.
Stumbled upon a unique article, I suggest you take a look https://1001viktorina.ru/forum/viewtopic.php?f=19&t=1099&sid=a5a3f47173eaa17866f2f1d2d9ada36c
Recently, I saw a discussion in a Facebook group about an interesting site with adult comics. I decided to check it out and landed on Mult34 – [url=https://mult34.com/family-values-sleepygimp/]sleepygimp[/url] . I was amazed by the variety of content and the quality of the materials.
I’ve revisited this site several times and always find something new. Mult34 – [url=https://mult34.com/category/zootopia/]zootopia porn[/url] is definitely worth a visit if you love erotic comics and cartoons. I highly recommend it to all my friends and acquaintances.
дом уборка
https://cleanprofispb.ru/
yoktogel
Inspirasi dari Ucapan Taylor Swift: Harapan dan Cinta dalam Lagu-Lagunya
Taylor Swift, seorang vokalis dan songwriter terkenal, tidak hanya dikenal karena lagu yang menawan dan vokal yang merdu, tetapi juga oleh karena lirik-lirik lagunya yang sarat makna. Pada lirik-liriknya, Swift sering menyajikan bermacam-macam aspek kehidupan, berawal dari asmara sampai dengan tantangan hidup. Di bawah ini adalah sejumlah ucapan motivatif dari lagu-lagunya, dengan artinya.
“Mungkin yang paling baik belum tiba.” – “All Too Well”
Penjelasan: Bahkan di masa-masa sulit, tetap ada seberkas asa dan kemungkinan akan hari yang lebih baik.
Lirik ini dari lagu “All Too Well” membuat kita ingat bahwa meskipun kita mungkin berhadapan dengan masa sulit saat ini, tetap ada peluang bahwa hari esok akan membawa sesuatu yang lebih baik. Situasi ini adalah pesan pengharapan yang menguatkan, memotivasi kita untuk tetap bertahan dan tidak menyerah, sebab yang terbaik mungkin belum datang.
“Aku akan tetap bertahan sebab aku tak bisa menjalankan apa pun tanpa kamu.” – “You Belong with Me”
Arti: Menemukan cinta dan support dari orang lain dapat menghadirkan kita daya dan niat untuk terus berjuang lewat tantangan.
горячая линия такси https://taxi-novocherkassk.ru
курсовые работы на заказ https://zakazat-kursovuyu-rabotu7.ru
На сайте https://migrant.support/poluchieniie_nomiera_pesel вы сможете оформить заявку для того, чтобы получить номер Песель по доверенности. Важным моментом является то, что вам не нужно приезжать в офис, потому как все работы могут быть выполнены онлайн. Такая услуга обойдется вам в 300 злотых. Всем оптовым покупателям предоставляется скидка, а покупка происходит на наиболее выгодных условиях. Выставляются фактуры vat. Документ высылается в день оформления, без опозданий и почтой
sirkuit4d
Ashley JKT48: Bintang yang Bercahaya Cemerlang di Kancah Idol
Siapa Ashley JKT48?
Siapa figur muda berbakat yang mencuri perhatian banyak penggemar lagu di Nusantara dan Asia Tenggara? Itulah Ashley Courtney Shintia, atau yang dikenal dengan nama bekennya, Ashley JKT48. Menjadi anggota dengan grup idola JKT48 pada tahun 2018, Ashley dengan cepat muncul sebagai salah satu anggota paling populer.
Biografi
Terlahir di Jakarta pada tanggal 13 Maret 2000, Ashley berketurunan darah Tionghoa-Indonesia. Beliau mengawali karier di dunia hiburan sebagai peraga dan pemeran, sebelum selanjutnya menjadi anggota dengan JKT48. Kepribadiannya yang ceria, suara yang bertenaga, dan kemampuan menari yang mengesankan menjadikannya idola yang sangat dicintai.
Award dan Pengakuan
Ketenaran Ashley telah diakui melalui berbagai award dan nominasi. Pada tahun 2021, beliau mendapat penghargaan “Anggota Paling Populer JKT48” di ajang JKT48 Music Awards. Ashley juga dianugerahi sebagai “Idol Tercantik se-Asia” oleh sebuah media online pada tahun 2020.
Peran dalam JKT48
Ashley memainkan fungsi penting dalam grup JKT48. Ia adalah member Tim KIII dan berperan sebagai penari utama dan vokalis. Ashley juga terlibat sebagai bagian dari unit sub “J3K” dengan Jessica Veranda dan Jennifer Rachel Natasya.
Perjalanan Mandiri
Selain aktivitasnya di JKT48, Ashley juga merintis karier individu. Ia telah merilis sejumlah single, termasuk “Myself” (2021) dan “Falling Down” (2022). Ashley juga telah bekerjasama dengan penyanyi lain, seperti Afgan dan Rossa.
Aktivitas Pribadi
Di luar dunia panggung, Ashley dikenal sebagai sebagai sosok yang rendah hati dan friendly. Beliau suka menyisihkan waktu bersama sanak famili dan sahabat-sahabatnya. Ashley juga punya kegemaran mewarnai dan fotografi.
БроМод https://bromod.ru/ – MODный BRO Market. Андроид APK моды и премиум игры. Здесь ты найдёшь оригинальные и МОДные приложения на свой Андроид апарат! БроМод – это Аналог Play Market с прямыми ссылками и бесплатными приложениями! Каждый пользователь найдет что-то свое. Для выбора доступно большое количество игр: разобраться в них поможет удобный фильтр поиска. Не меньше чем несколько сотен страниц с онлайн-шутерами, головоломками и другими игрушками размещены на сайте русскоязычной версии Play Market.
проверить usdt erc20 15
Проверка адреса криптовалюты
Проверка USDT на блокчейне TRC20 и других криптовалютных операций
На нашем портале представлены развернутые обзоры многочисленных инструментов для проверки операций и счетов, включая антиотмывочного закона контроли для монет и прочих цифровых валют. Вот главные функции, представленные в наших обзорах:
Анализ криптовалюты на сети TRC20
Многие инструменты обеспечивают комплексную анализ транзакций криптовалюты на платформе TRC20 сети. Это гарантирует обнаруживать подозреваемую действия и соответствовать законодательным требованиям.
Контроль платежей токенов
В представленных ревью указаны инструменты для глубокого проверки и отслеживания транзакций токенов, которые обеспечивает обеспечивать ясность и защищенность переводов.
антиотмывочная контроль криптовалюты
Известные инструменты предлагают anti-money laundering анализ криптовалюты, давая возможность фиксировать и исключать ситуации финансовых преступлений и валютных преступлений.
Анализ аккаунта токенов
Наши описания охватывают ресурсы, предназначенные для обеспечивают анализировать счета USDT на предмет подозрительных действий и сомнительных операций, предоставляя повышенную степень безопасности.
Контроль платежей USDT на сети TRC20
В наших обзорах описаны ресурсы, обеспечивающие проверку платежей токенов на блокчейне TRC20 блокчейна, что обеспечивает соответствие соблюдение всем стандартам положениям.
Верификация счета счета криптовалюты
В описаниях указаны сервисы для проверки аккаунтов аккаунтов криптовалюты на потенциальных угроз угроз.
Верификация кошелька токенов на блокчейне TRC20
Наши ревью представляют ресурсы, поддерживающие анализ счетов монет на блокчейне TRC20 блокчейна, что предотвращает помогает исключение финансовых преступлений и экономических преступлений.
Контроль USDT на легитимность
Описанные инструменты позволяют контролировать платежи и аккаунты на чистоту, фиксируя сомнительную действия.
антиотмывочного закона проверка монет на блокчейне TRC20
В описаниях описаны ресурсы, обеспечивающие anti-money laundering проверку для криптовалюты на блокчейне TRC20 сети, что помогает вашему делу выполнять международным положениям.
Анализ USDT ERC20
Наши ревью представляют платформы, обеспечивающие верификацию USDT на блокчейне ERC20 сети, что гарантирует проведение проведение транзакций и счетов.
Анализ виртуального кошелька
Мы изучаем платформы, поддерживающие опции по анализу криптовалютных кошельков, включая контроль переводов и фиксирование подозрительной действий.
Проверка адреса цифрового кошелька
Наши обзоры представляют платформы, позволяющие контролировать счета криптокошельков для поддержания дополнительной степени надежности.
Контроль виртуального кошелька на операции
В наших обзорах доступны сервисы для анализа криптовалютных кошельков на платежи, что помогает помогает поддерживать открытость переводов.
Контроль виртуального кошелька на прозрачность
Наши ревью представляют платформы, дающие возможность проверять криптовалютные кошельки на чистоту, выявляя любые необычные активности.
Читая подробные оценки, вам удастся подберете надежные платформы для верификации и мониторинга виртуальных платежей, для поддерживать повышенный степень безопасности защиты и соблюдать всех нормативным правилам.
заказ такси недорого такси город телефон
Igrosite – портал для геймеров, который предоставляет большой спектр контента, включая обзоры, статьи и руководства, а также новости. Здесь можно выбрать игры для своей коллекции и общаться с игроками в дружеской атмосфере, узнать последние события в мире игр. https://igrosite.ru – сайт, где мы подготовили для вас интервью с разработчиками, аналитические материалы и факты, чтобы вы могли заглянуть за кулисы создания игр. Узнаете интересные подробности о развитии этой увлекательной отрасли. Посетите прямо сейчас наш портал!
Сайт ukrayinka.com.ua пользуется невероятной популярностью и станет вам верным другом. Мы освещаем экономические и общественно-политические события в Украине. Предлагаем только качественную информацию. ukrayinka.com.ua – https://ukrayinka.com.ua сайт, который понятен любому пользователю. Здесь вы найдете статьи с хорошими фото. Рапологайтесь поудобнее и читайте новости. Готовы обеспечить вас всеми необходимыми сведениями. Смело выбирайте наш портал, если цените наполненность и свежесть. Вы не пожалеете об этом!
Online Casinos: Innovation and Benefits for Modern Society
Introduction
Online gambling platforms are virtual sites that offer players the opportunity to participate in betting activities such as poker, spin games, blackjack, and slot machines. Over the past few years, they have become an essential component of online leisure, providing various benefits and opportunities for players around the world.
Availability and Ease
One of the main advantages of online gambling sites is their accessibility. Players can play their favorite games from anywhere in the world using a computer, iPad, or smartphone. This saves time and money that would typically be used going to land-based casinos. Additionally, round-the-clock availability to activities makes online casinos a easy choice for individuals with hectic schedules.
Range of Games and Entertainment
Digital casinos provide a vast variety of activities, allowing everyone to discover an option they enjoy. From classic table games and board games to slot machines with various themes and progressive jackpots, the range of activities guarantees there is an option for every preference. The ability to engage at various proficiencies also makes digital gambling sites an perfect place for both beginners and seasoned players.
Economic Benefits
The digital gambling sector contributes significantly to the economy by creating jobs and producing revenue. It supports a diverse range of professions, including software developers, customer support agents, and marketing professionals. The income generated by online gambling sites also contributes to government funds, which can be allocated to support public services and infrastructure initiatives.
Technological Innovation
Digital gambling sites are at the forefront of technological advancement, continuously integrating new technologies to improve the playing experience. High-quality visuals, real-time dealer games, and virtual reality (VR) gambling sites offer engaging and realistic playing entertainment. These innovations not only enhance player experience but also push the boundaries of what is achievable in online leisure.
Responsible Gambling and Assistance
Many online gambling sites promote responsible gambling by offering tools and assistance to help users control their betting activities. Features such as fund restrictions, self-exclusion choices, and access to assistance programs guarantee that users can enjoy gaming in a safe and monitored environment. These measures show the industry’s dedication to encouraging healthy betting habits.
Social Interaction and Community
Online casinos often provide interactive options that allow players to connect with each other, forming a feeling of community. Multiplayer activities, communication tools, and networking integration enable players to connect, exchange experiences, and form friendships. This interactive element improves the entire gaming experience and can be especially helpful for those seeking community engagement.
Summary
Digital casinos offer a diverse variety of advantages, from availability and ease to economic contributions and innovations. They offer varied gaming options, encourage responsible gambling, and promote community engagement. As the sector continues to evolve, digital casinos will likely stay a major and positive presence in the world of online entertainment.
free slots games
No-Cost Slot Games: Fun and Advantages for All
Complimentary slot games have become a in-demand form of online fun, granting players the rush of slot machines without any economic investment.
The primary purpose of no-cost slot games is to deliver a fun and immersive way for players to relish the rush of slot machines absent any financial liability. They are conceived to mimic the sensation of for-profit slots, allowing players to rotate the reels, savor various ideas, and earn electronic winnings.
Fun: Complimentary slot games are an excellent source of fun, providing hours of pleasure. They feature lively illustrations, immersive audio, and varied themes that serve a comprehensive array of tastes.
Skill Development: For beginners, complimentary slot games offer a risk-free setting to acquaint the mechanics of slot machines. Players can get acquainted with multiple feature sets, win lines, and special features absent the worry of losing funds.
Destressing: Playing complimentary slot games can be a great way to decompress. The simple handling and the chance for virtual winnings make it an satisfying hobby.
Social Interaction: Many complimentary slot games incorporate collaborative features such as tournaments and the ability to engage with friends. These features bring a collective layer to the gaming experience, motivating players to pit themselves against one another.
Rewards of No-Cost Slot Games
1. Approachability and Ease
No-Cost slot games are conveniently accessible to anyone with an network connection. They can be experienced on multiple gadgets including computers, pads, and handsets. This simplicity enables players to relish their favorite offerings regardless of time and from any place.
2. Economic Risk-Freeness
One of the principal perks of gratis slot games is that they eradicate the economic risks linked to gaming. Players can experience the suspense of activating the reels and earning major payouts devoid of risking any capital.
3. Breadth of Offerings
No-Cost slot games are presented in a broad array of concepts and configurations, from traditional fruit slots to modern slot machines with video with complex themes and imagery. This variety guarantees that there is an alternative for all, irrespective of their tastes.
4. Developing Intellectual Aptitudes
Playing no-cost slot games can help improve mental capabilities such as strategic thinking. The requirement to understand paylines, learn functional concepts, and predict effects can offer a cognitive challenge that is concurrently pleasurable and useful.
5. Risk-Free Trial Phase for Actual-Currency Gaming
For those contemplating transitioning to actual-currency slots, free slot games grant a beneficial pre-experience. Players can experiment with diverse games, hone approaches, and acquire confidence in advance of electing to wager real capital. This preparation can translate to a more knowledgeable and satisfying actual-currency gaming encounter.
Recap
Gratis slot games deliver a multitude of perks, from absolute pleasure to skill development and social interaction. They offer a safe and cost-free way to savor the excitement of slot machines, rendering them a valuable enhancement to the world of electronic recreation. Whether you’re looking to relax, sharpen your thinking abilities, or just derive entertainment, free slot games are a superb alternative that steadfastly captivate players around.
slots machines
No-Cost Slot-Based Games: Amusement and Perks for Individuals
Introduction
Slot-based activities have historically been a fixture of the casino experience, delivering customers the prospect to secure major payouts with only the trigger of a arm or the push of a control. In the modern era, slot machines have as well transformed into widespread in internet-based gaming sites, making them reachable to an even more broader set of users.
Amusement Factor
Slot-related offerings are crafted to be entertaining and absorbing. They feature animated graphics, electrifying sound effects, and wide-ranging ideas that suit a extensive array of inclinations. Regardless of whether players relish traditional fruit-related symbols, thrill-based slot-related offerings, or slots based on iconic movies, there is an option for anyone. This range ensures that players can always discover a experience that fits their inclinations, providing durations of fun.
Simple to Engage With
One of the biggest positives of slot-related offerings is their uncomplicated nature. In contrast to certain casino games that call for forethought, slot-related offerings are straightforward to grasp. This renders them available to a extensive set of users, incorporating newcomers who may feel discouraged by more sophisticated experiences. The easy-to-grasp essence of slot-related offerings permits customers to relax and enjoy the experience absent fretting about complex guidelines.
Unwinding and Destressing
Partaking in slot machines can be a wonderful way to decompress. The monotonous quality of activating the drums can be tranquil, delivering a mental reprieve from the demands of daily experience. The chance for obtaining, even if it constitutes merely small sums, injects an aspect of suspense that can elevate participants’ moods. Several individuals find that interacting with slot-based games facilitates them destress and divert their attention from their concerns.
Communal Engagement
Slot machines likewise present avenues for social participation. In land-based gaming venues, players often congregate in proximity to slot-based activities, encouraging their fellow players on and rejoicing in wins collectively. Online slots have as well included social elements, such as leaderboards, allowing participants to network with peers and exchange their interactions. This feeling of shared experience improves the overall interactive experience and can be especially enjoyable for people seeking social participation.
Monetary Upsides
The widespread appeal of slot-based games has substantial monetary rewards. The sector generates opportunities for game engineers, casino personnel, and customer support agents. Moreover, the income obtained by slot machines provides to the fiscal landscape, granting revenue revenues that finance societal initiatives and facilities. This economic effect extends to equally land-based and online gaming venues, rendering slot machines a valuable component of the interactive industry.
Mental Upsides
Interacting with slot machines can in addition have cognitive benefits. The game calls for customers to make rapid determinations, detect patterns, and supervise their betting approaches. These cognitive activities can help preserve the mind focused and bolster cerebral faculties. In the case of mature players, partaking in cognitively stimulating activities like interacting with slot-based games can be beneficial for maintaining mental health.
Approachability and Simplicity
The emergence of virtual casinos has established slot machines additional approachable than ever. Customers can experience their favorite slot-related offerings from the simplicity of their private homes, using laptops, tablets, or mobile phones. This user-friendliness allows individuals to partake in at any time and irrespective of location they want, absent the need to commute to a physical gambling establishment. The availability of gratis slot-based games in addition allows participants to enjoy the experience free from any financial investment, establishing it an open-to-all style of fun.
Conclusion
Slot-related offerings deliver a wealth of upsides to users, from sheer amusement to mental benefits and communal engagement. They provide a secure and zero-cost way to savor the excitement of slot-based activities, making them a valuable complement to the realm of digital recreation.
Whether you’re seeking to decompress, sharpen your cerebral aptitudes, or simply have fun, slot-related offerings are a superb option that persistently captivate customers across.
Significant Advantages:
– Slot machines grant entertainment through lively imagery, engaging audio, and varied motifs
– Ease of play establishes slot-related offerings approachable to a extensive set of users
– Playing slot-based activities can deliver stress relief and cerebral upsides
– Group-based functions improve the total gaming experience
– Internet-based availability and no-cost possibilities make slot-based activities inclusive kinds of entertainment
In recap, slot machines steadfastly provide a diverse collection of benefits that suit players throughout. Whether desiring pure entertainment, intellectual engagement, or group-based involvement, slot-based activities stay a fantastic alternative in the constantly-changing domain of digital recreation.
курсовые работы на заказ https://zakazat-kontrolnuyu7.ru
Luck Wagering Environment: In a Place Where Pleasure Combines With Wealth
Luck Gaming Site is a renowned internet-based place known for its comprehensive variety of games and enthralling bonuses. Let’s investigate the motivations behind so a significant number of individuals experience playing at Prosperity Gaming Site and the extent to which it benefits them.
Entertainment Value
Fortune Casino provides a variety of games, including classic wagering games like blackjack and roulette, as well as innovative slot-based games. This breadth ensures that there is something for all, establishing each trip to Luck Wagering Environment satisfying and fun.
Substantial Payouts
One of the principal draws of Fortune Casino is the opportunity to earn significant rewards. With generous major payouts and incentives, participants have the opportunity to achieve unexpected success with a single play or game. Many participants have obtained considerable payouts, enhancing the excitement of playing at Luck Gambling Platform.
User-Friendliness and Availability
Prosperity Wagering Environment’s internet-based interface establishes it as user-friendly for participants to savor their most liked experiences from anywhere. Regardless of whether at dwelling or in transit, users can engage with Fortune Wagering Environment from their PC or mobile device. This reachability provides that customers can savor the anticipation of the gambling regardless of when they desire, without the need to journey.
Variety of Games
Wealth Wagering Environment presents a broad assortment of experiences, securing that there is an alternative for every single style of customer. Starting with established casino games to story-based slot machines, the diversity retains players engaged and delighted. This array as well enables participants to experiment with new experiences and identify unfamiliar most cherished.
Bonuses and Rewards
Wealth Wagering Environment acknowledges its players with bonuses and rewards, involving introductory bonuses and loyalty programs. These special offers not merely improve the entertainment interaction but likewise raise the opportunities of achieving substantial winnings. Players are constantly incentivized to sustain interaction, establishing Fortune Casino even more appealing.
Communal Engagement and Interpersonal Connections
ChatGPT l Валли, [06.06.2024 4:30]
Wealth Gaming Site delivers a feeling of collective engagement and communal engagement for users. Via chat rooms and interactive platforms, participants can connect with their peers, communicate strategies and methods, and in certain cases create interpersonal bonds. This collaborative aspect contributes a further dimension of enjoyment to the leisure interaction.
Summary
Prosperity Gambling Platform grants a broad array of benefits for participants, including entertainment, the likelihood of earning significant rewards, ease, breadth, rewards, and interpersonal connections. Whether desiring thrill or hoping to strike it rich, Wealth Wagering Environment grants an enthralling sensation for everyone who partake in.
На сайте https://stroyengr.ru/ получите всю актуальную, интересную информацию, которая касается недвижимости, налоговых вычетов, финансов, ипотеки, продажи квартиры, материнского капитала, налогов и многого другого. Все материалы с поправкой на обновления, а потому вы можете ориентироваться на этот контент. Обязательно побывайте в разделе «Свежие записи», где вы найдете не менее интересный и любопытный контент. Вы узнаете и о том, как правильно оформить дарственную, про налоговый вычет.
free poker machine games
No-Cost Electronic Gaming Games: A Enjoyable and Beneficial Experience
Gratis slot-based experiences have transformed into steadily widely-accepted among customers aiming for a thrilling and secure gaming interaction. These games offer a broad selection of benefits, establishing them as a favored option for a significant number of. Let’s explore in which manner free poker machine games can reward players and the motivations behind they are so extensively relished.
Amusement Factor
One of the principal reasons individuals savor engaging with complimentary slot-based games is for the amusement factor they provide. These activities are created to be engaging and thrilling, with lively visuals and engrossing music that bolster the holistic gaming interaction. Whether you’re a recreational player wanting to spend time or a enthusiastic gaming aficionado aiming for suspense, complimentary slot-based offerings provide amusement for everyone who.
Competency Enhancement
Interacting with gratis electronic gaming games can in addition enable hone helpful aptitudes such as strategic thinking. These offerings require players to render rapid determinations contingent on the virtual assets they are dealt, assisting them enhance their analytical skills and intellectual prowess. Additionally, customers can try out various strategies, refining their skills absent the chance of negative outcome of losing paid funds.
User-Friendliness and Availability
An additional reward of free poker machine games is their user-friendliness and reachability. These activities can be played in the virtual sphere from the ease of your own home, excluding the need to make trips to a traditional casino. They are likewise available 24/7, permitting users to relish them at any desired moment that fits them. This user-friendliness makes gratis electronic gaming offerings a popular choice for players with demanding agendas or those looking for a quick entertainment fix.
Communal Engagement
A significant number of free poker machine offerings as well present collaborative features that enable users to communicate with their peers. This can feature messaging platforms, forums, and multiplayer settings where participants can go up against their peers. These social interactions inject an additional layer of enjoyment to the gaming interaction, permitting players to connect with like-minded individuals who display their interests.
Tension Alleviation and Psychological Rejuvenation
Playing no-cost virtual wagering activities can as well be a excellent method to decompress and unwind after a prolonged period. The effortless gameplay and calming audio can facilitate lower anxiety and nervousness, delivering a welcome escape from the challenges of normal existence. Furthermore, the thrill of winning online credits can enhance your disposition and leave you feeling rejuvenated.
Recap
Complimentary slot-based games provide a broad selection of upsides for players, involving entertainment, proficiency improvement, convenience, social interaction, and stress relief and unwinding. Regardless of whether you’re seeking to enhance your leisure abilities or simply enjoy yourself, no-cost virtual wagering activities grant a rewarding and pleasurable encounter for participants of any levels.
Digital Card Games: A Wellspring of Amusement and Competency Enhancement
Virtual casino-style games has arisen as a well-liked form of fun and a platform for proficiency improvement for customers across the globe. This article explores the constructive facets of virtual casino-style games and the extent to which it benefits users, underscoring its far-reaching acceptance and influence.
Amusement Factor
Online poker provides a thrilling and absorbing entertainment sensation, enthralling customers with its thoughtful gameplay and unpredictable ends. The experience’s immersive character, along with its communal aspects, grants a unique form of fun that several regard as satisfying.
Proficiency Improvement
Beyond fun, virtual casino-style games as well acts as a channel for skill development. The game requires problem-solving, decision-making under pressure, and the aptitude to comprehend rivals, all of these add to cognitive development. Customers can bolster their critical-thinking faculties, self-awareness, and risk management skills through regular gameplay.
User-Friendliness and Availability
One of the primary rewards of virtual casino-style games is its ease and reachability. Participants can savor the experience from the ease of their abodes, at any moment that aligns with them. This availability eradicates the need for commute to a land-based wagering facility, rendering it a convenient option for individuals with hectic timetables.
Diversity of Options and Bet Sizes
Internet-based card games systems present a extensive variety of offerings and wagers to target customers of every stages of proficiency and tastes. Regardless of whether you’re a novice looking to grasp the basics or a seasoned expert aiming for a test, there is a offering for your preferences. This diversity guarantees that participants can constantly uncover a game that matches their skill level and bankroll.
Interpersonal Connections
Internet-based card games likewise delivers opportunities for social interaction. Several infrastructures present communication tools and multiplayer settings that permit users to communicate with others, discuss sensations, and form social relationships. This social element injects depth to the gaming sensation, making it further enjoyable.
Monetary Gains
For those inclined, digital table games can in addition be a provider of financial rewards. Adept players can obtain major profits through consistent gameplay, establishing it as a financially rewarding undertaking for those who excel at the experience. Furthermore, several digital table games matches provide significant prize pools, granting players with the prospect to earn significant rewards.
Recap
Online poker presents a range of advantages for users, incorporating amusement, skill development, user-friendliness, social interaction, and monetary gains. Its popularity steadfastly grow, with several players opting for internet-based card games as a origin of enjoyment and development. Whether you’re seeking to enhance your aptitudes or just derive entertainment, internet-based card games is a multifaceted and profitable leisure activity for users of every experiences.
решение задач на заказ https://resheniye-zadach7.ru заказать онлайн
https://mashable.com/article/artist-vandalizes-snapchat-ar-jeff-coons-collab
Opened up an intriguing read – let me share this with you http://chatruletka.iwopop.com
На сайте https://autopiter.ru/ закажите как оригинальные, так и неоригинальные запчасти, изучите каталоги ВАЗ, КАМАЗ, ГАЗ. Также здесь представлены автомасла, химия, аккумуляторы, все для спецтехники и многое другое. Вся продукция от именитых, лидирующих брендов, которые отвечают за качество. На продукцию установлены доступные цены, действует оперативная доставка. У всех масел имеется гарантия качества. При необходимости можно воспользоваться онлайн-поиском фильтров. Заказы принимают, обрабатывают каждый день.
На сайте http://nemans.ru вы сможете приобрести всю необходимую продукцию, включая пакеты с ручками, фасовочные пакеты, мешки полипропиленовые, пленку армированную, полиэтиленовую. Также в разделе имеется ветошь, ароматное мыло, перчатки, черенки, бытовая химия, ведра и многое другое, что вы сможете заказать прямо сейчас. Среди рекомендованных товаров находятся вязаные перчатки, туалетная бумага, хозяйственное мыло, неткол. Вся продукция отличного качества, надежная и наделена длительными сроками эксплуатации.
рефераты на заказ https://kupit-referat213.ru
https://bicrypto.exchange – crypto exchange software. White label, open-source exchange solution with a focus on a super-fast, pixel-perfect interface and robust security. High-performance platform with a robust internal architecture. Leverages the capabilities of Nuxt3 to create a cutting-edge user interface.
аюваска купить кратом купить в москве
Популярный интернет-магазин CLAPS предлагает купить по доступной цене DreamStalker Expert. Прибор в лобной части маски находится, особая конструкция обеспечивает вентиляцию, отсутствие запотевание лба, комфортный сон и охлаждение. https://claps.me – сайт, где имеется каталог, в нем есть описание, характеристики, фотографии товара. Тут предложены отзывы радостных клиентов, с которыми вы можете ознакомиться уже сейчас. Также на портале найдете к DreamStalker Expert инструкции.
Безопасная сухая химия для вашей любимой мебели
Cухая химчистка мягкой мебели [url=https://suhaya-himchistka-mebely.ru]https://suhaya-himchistka-mebely.ru[/url] .
Как найти надежную компанию для шумопоглощения в автомобиле?
Проклейка автомобиля шумоизоляцией цена [url=https://www.shumoizolyaciya-a.ru/]https://www.shumoizolyaciya-a.ru/[/url] .
Индивидуальный подход к каждому клиенту, безупречный результат
Полировка кузова авто стоимость [url=https://polish-avto.ru/]https://polish-avto.ru/[/url] .
https://kkc-cleaning.ru/
Discovered an interesting article, I suggest you familiarize yourself https://dazzle.ru/uslugi/11937-chto-nuzhno-muzhchinam-ot-elitnyh-eskortnic.html
Завод «СХТ» предлагает лучшее весоизмерительное оборудование. В своем составе имеем собственную сервисную службу и метрологическую лабораторию. Проводим поверку более 2000 единиц автомобильных весов в год! https://xn--q1aci.xn--p1ai/ – сайт, где вы можете узнать о том, каким нынешний весоизмерительный комплекс должен быть. Здесь есть фотогалерея и отзывы довольных клиентов. Можете заказать у нас коллейные, электронные, переносные автомобильные весы. Мы оперативно реагируем на заявки и даем консультации по необходимым вопросам, обращайтесь.
уборка в частном доме
unoslot
Unduh Perangkat Lunak 888 dan Raih Hadiah: Manual Cepat
**Perangkat Lunak 888 adalah alternatif terbaik untuk Pengguna yang menginginkan aktivitas berjudi online yang seru dan bernilai. Dengan imbalan harian dan fasilitas menarik, perangkat lunak ini menawarkan menawarkan keseruan bermain unggulan. Inilah manual singkat untuk memanfaatkan pemanfaatan App 888.
Pasang dan Mulailah Raih
Platform Terdapat:
Perangkat Lunak 888 bisa diambil di Perangkat Android, iOS, dan Komputer. Mulai main dengan mudah di perangkat apapun.
Imbalan Tiap Hari dan Bonus
Bonus Mendaftar Harian:
Login setiap periode untuk mengambil bonus sampai 100K pada hari ketujuh.
Tuntaskan Aktivitas:
Raih peluang undian dengan menyelesaikan tugas terkait. Masing-masing pekerjaan memberi Anda satu peluang undian untuk mendapatkan imbalan hingga 888K.
Pengklaiman Sendiri:
Keuntungan harus diklaim mandiri di dalam program. Pastikan untuk meraih hadiah saban masa agar tidak kadaluwarsa.
Prosedur Lotere
Peluang Undian:
Setiap waktu, Anda bisa mendapatkan satu kesempatan pengeretan dengan menuntaskan pekerjaan.
Jika opsi undian habis, rampungkan lebih banyak misi untuk mengklaim tambahan opsi.
Tingkat Imbalan:
Raih bonus jika jumlah undian Para Pengguna lebih dari 100K dalam waktu satu hari.
Aturan Utama
Pengklaiman Imbalan:
Bonus harus diterima manual dari aplikasi. Jika tidak, imbalan akan langsung dikreditkan ke akun pengguna Kamu setelah satu hari.
Persyaratan Pertaruhan:
Hadiah butuh setidaknya satu pertaruhan berlaku untuk digunakan.
Kesimpulan
Program 888 menghadirkan aktivitas main yang mengasyikkan dengan keuntungan signifikan. Unduh perangkat lunak hari ini dan nikmati kemenangan besar setiap waktu!
Untuk informasi lebih lanjut tentang promo, pengisian, dan program rujukan, lihat laman home aplikasi.
Новинки музыки https://muzlada.net/ за сегодня в mp3, Хиты России и Украины. Треки как самых популярных, так и малоизвестных исполнителей. Слушайте треки и скачивайте их на свой ПК или телефон. У нас вы найдёте самые свежие новинки песен в хорошем качестве. На сайте собрана огромная коллекция композиций разных направлений, ведь мы хотим, чтобы каждый нашёл то, что ему нужно. В каталоге представлена как русская музыка, так и зарубежные хиты. Любой из них вы сможете скачать абсолютно бесплатно в формате MP3.
https://uborkadoma-spb.ru/
Вас интересует прокат инвалидных колясок? Деливери плей поможет вам. Гарантируем доступные цены и быструю доставку. У нас коляски исключительно чистые. Вы сможете, заказ оплатить тогда, когда убедитесь в том, что коляска находится в отменном состоянии и полностью соответствует вашим требованиям. Ищите Инвалидная коляска Напрокат? Deliveryplay.ru/arenda-invalidnyh-kolyasok – сайт, где можете арендовать коляску. Также здесь вы узнаете, почему аренда лучше покупки. Мы поможем вам выбрать идеальную коляску, которая вам подойдет, обращайтесь.
На сайте https://rozmarysochi.ru/ забронируйте номер в семейном отеле. Для этого необходимо обозначить дату заезда, выезда, а также количество гостей и другую важную информацию. Это место для тех, кто не готов на компромиссы, а ценит удобство, комфорт и качество во всем исполнении. Отпуск подарит огромное количество приятных, положительных эмоций, ярких впечатлений. Здесь можно отдохнуть и с детьми, а также большой компанией. Регулярно проходят акции. Имеется теннисный корт, детский клуб и многое другое.
Любите слушать в отменном качестве радио онлайн? Топ Волна предоставляет такую возможность. У нас есть Европа Плюс, Юмор FM, авторадио, DFM и другое. Любую радиостанцию можете слушать дома, в дороге и на работе. Вы будете в курсе новых композиций и последних новостей. top-volna.ru – https://top-volna.ru здесь собрали радиостанции различных направлений и музыкальных жанров. Найдите нужную волну и получайте удовольствие от любимых мелодий. Можете оставить свой отзыв на выбранную радиостанцию, вам нужно перейти в конец страницы и нажать на соответствующую кнопку.
купить диплом техника http://www.6landik-diploms.com
На сайте https://ack-group.ru/stati-po-psikhologii/psikhoterapiya/ вы сможете получить всю необходимую информацию, которая касается психотерапии. Перед вами огромное количество статей, с которыми необходимо ознакомиться каждому, кто хочет улучшить свое психологическое состояние и получить ценные и редкие советы от профессионалов. Психотерапия предполагает использование самых разных методик, которые помогут себя хорошо чувствовать и ощутить гармонию с окружающей средой. Под психотерапией понимают активное воздействие на психику человека.
сколько стоит сайдинг цена купить виниловый сайдинг
безопасно,
Современное оборудование и материалы, для крепких и здоровых зубов,
Профессиональное лечение и консультации, для вашего уверенного выбора,
Комфортные условия и дружественный персонал, для вашего здоровья и благополучия,
Эффективное лечение зубов и десен, для вашего здоровья и красоты улыбки,
Профессиональная гигиена полости рта, для вашего долгосрочного удовлетворения,
Современное лечение заболеваний полости рта, для вашего комфорта и удовлетворения
послуги стоматології [url=https://stomatologichnaklinikafghy.ivano-frankivsk.ua/]послуги стоматології[/url] .
twin casino сайт Twin Casino
Желаете приобрести по недорогой стоимости в Донецке гранитный памятник? Каменный двор – компания, которая предлагает качественные услуги. Сотрудники применяют новейшие технологии и оборудование, что дает возможность быстро и точно исполнить заказ. https://kamdvor.ru – сайт, где можно подробнее узнать об акциях и скидках. Здесь вы найдете каталог, который можно посмотреть уже сейчас. Мы относимся к своим клиентам с уважением и гарантируем доступные цены на памятники. Позвоните нам, мы проконсультируем вас бесплатно.
Came across a unique article Ц it’s worth your attention https://www.sonicsrising.com/users/worksale555
Если вам нужен потребительский кредит, канал [url=https://t.me/s/porteb_kredit]Потребительские кредиты – вся информация[/url] – ваш лучший помощник. Мы собрали все предложения банков, рассказали о рисках и нюансах. Удобно, просто и информативно. Подписывайтесь и будьте финансово грамотными!
парма цены https://messir-zakaz.ru
HP/HPE коммутаторы Коммутаторы от HP/HPE для высокоскоростных сетей.
Ищите срочный вывоз мусора в Москве? Обратитесь к нам https://vivoz-musora.pro/ и мы приедем в течении 50 минут. Бесплатная консультация по вывозу и утилизации или выберете на сайте необходимую услугу по вывозу отходов, а мы предложим лучшие условия. Мы работаем круглосуточно. Подробнее на сайте.
Все самое интересное из мира игр https://unionbattle.ru обзоры, статьи и ответы на вопросы
https://cleanb2b.ru/
Зайдите на сайт ФильтрМаркет https://filtrmarket.ru/ где большой каталог фильтров, которые сможете купить с доставкой – воздушные фильтры, магистральные, панельные, масляные фильтры. Все в наличие по отличным ценам в едином каталоге. Подробнее на сайте.
На сайте https://hotel-elizabeth.ru/ каждый желающий получает возможность забронировать отель «Элизабет». Укажите дату заезда, выезда, количество гостей и многое другое. А если у вас есть промокод, то введите комбинацию чисел, чтобы сэкономить. Вас ожидает удивительный чистый воздух, роскошные горы, а также привлекательные виды, которыми можно наслаждаться повсеместно. Установлены доступные расценки, а огромное количество развлекательных программ уже входит в стоимость.
poker game free
Unveiling the Universe of Complimentary Poker
Beginning
In contemporary times, the game of poker have transformed into extensively available entertainment options. For those wanting a free means to play poker games, no-cost poker platforms supply a thrilling adventure. This piece examines the pros and factors as to why free poker games has become a popular choice for numerous enthusiasts.
Advantages of Free Poker Games
Cost-Free Entertainment
One of the most enticing aspects of complimentary poker is that it gives gamers with complimentary fun. There is no necessity to use cash to play the card game, making it attainable to all.
Skill Development
Engaging in complimentary poker permits players to hone their prowess without one financial danger. It is a perfect opportunity for learners to get the rules and approaches of poker.
Social Engagement
Many no-cost poker applications offer possibilities for group connection. Enthusiasts can connect with others, discuss techniques, and experience friendly games.
Why Many Players Prefer Poker Game Free
Accessibility
Free poker games are widely accessible, allowing enthusiasts from diverse locations to engage in the card game.
No Fiscal Risk
With complimentary poker, there is no economic risk, making it a risk-free choice for users who wish to experience poker without spending currency.
Variety of Games
No-cost poker platforms supply a extensive array of games, ensuring that users can continually find an option that aligns with their likes.
Final Thoughts
Free poker games provides a enjoyable and reachable method for users to play poker. With zero monetary risk, options for improving skills, and diverse game choices, it is understandable that various users like complimentary poker as their preferred gambling choice.
no deposit bonus
Online gambling sites are becoming more popular, delivering different incentives to attract newcomers. One of the most attractive opportunities is the no deposit bonus, a promotion that lets gamblers to try their luck without any initial deposit. This article discusses the advantages of no deposit bonuses and emphasizes how they can enhance their value.
What is a No Deposit Bonus?
A no-deposit bonus is a form of casino offer where gamblers are granted bonus funds or free spins without the need to put in any of their own cash. This permits players to test the online casino, experiment with diverse game options and possibly win real cash, all without any monetary input.
Advantages of No Deposit Bonuses
Risk-Free Exploration
No deposit bonuses provide a cost-free chance to explore online gambling sites. Gamblers can evaluate multiple games, familiarize themselves with the gaming environment, and judge the overall gameplay without utilizing their own money. This is significantly advantageous for new players who may not be familiar with online casinos.
Chance to Win Real Money
One of the most appealing benefits of free bonuses is the chance to win real money. While the amounts may be modest, any gains earned from the bonus can often be redeemed after meeting the casino’s staking criteria. This adds an element of excitement and gives a prospective financial gain without any monetary outlay.
Learning Opportunity
No-deposit bonuses give a excellent opportunity to learn how multiple casino games function. Players can experiment with tactics, learn the mechanics of the games, and develop into more proficient without being afraid of risking their own money. This can be especially beneficial for complex casino games like poker.
Conclusion
No deposit bonuses give several upsides for participants, such as secure trial, the possibility to win real money, and valuable development experiences. As the market keeps to develop, the demand of no upfront deposit bonuses is anticipated to rise.
https://earl-clean.ru/
Exploring Lottery Casinos: A Captivating and Reachable Playing Possibility
Introduction
Lottery casinos are transforming into a favored substitute for users seeking an exciting and lawful way to partake in online betting. Differing from traditional online gaming hubs, sweepstakes gambling platforms work under separate lawful models, allowing them to deliver competitions and awards without being subject to the same laws. This article explores the concept of sweepstakes gaming hubs, their merits, and why they are attracting a increasing number of participants.
What is a Sweepstakes Casino?
A lottery gambling platform works by providing gamers with online currency, which can be utilized to participate in events. Gamers can gain additional virtual coins or real prizes, like money. The primary distinction from standard gambling platforms is that users do not buy funds instantly but obtain it through promotional activities, like buying a product or engaging in a complimentary access promotion. This system permits sweepstakes betting sites to operate authorized in many jurisdictions where conventional virtual wagering is restricted.
real money slots
Unveiling Real Money Slots
Introduction
Money slots have grown into a popular selection for gamblers wanting the rush of securing genuine cash. This article explores the pros of cash slots and the causes they are gaining a growing number of enthusiasts.
Advantages of Cash Slots
Actual Payouts
The most appeal of real money slots is the possibility to secure real money. As opposed to no-cost slots, gambling slots offer enthusiasts the rush of prospective monetary payouts.
Wide Range of Games
Money slots offer a wide array of types, features, and payout structures. This makes sure that there is an activity for every kind of gambler, including old-school classic 3-reel slots to up-to-date animated slots with multiple winning lines and additional features.
Exciting Bonuses and Promotions
Numerous online casinos give enticing rewards for money slot users. These can include sign-up rewards, free spins, rebate offers, and loyalty programs. Such incentives increase the overall playing experience and supply further chances to earn money.
Why Players Choose Real Money Slots
The Adrenaline of Gaining Genuine Funds
Cash slots offer an thrilling journey, as gamblers await the potential of winning actual cash. This characteristic contributes a further degree of thrill to the gaming journey.
Prompt Payouts
Money slots supply enthusiasts the reward of prompt payments. Earning funds quickly improves the playing journey, making it more rewarding.
Extensive Game Variety
Alongside money slots, users can play a broad selection of games, ensuring that there is constantly an activity different to play.
Final Thoughts
Cash slots offers a exhilarating and satisfying playing activity. With the possibility to earn tangible funds, a wide selection of slot machines, and enticing offers, it’s no wonder that numerous gamblers like real money slots for their betting requirements.
На сайте https://sobr26.ru ознакомьтесь с номером телефона охранной организации СОБР. Здесь оказываются услуги, связанные с охраной недвижимости, организуется безопасность объекта, есть возможность воспользоваться физической охраной, которая оказывается более 20 лет. Прямо сейчас вы сможете рассчитывать на бесплатную консультацию. Каждый клиент сможет организовать пультовую, физическую охрану, пожарную сигнализацию, видеонаблюдение. Для того чтобы воспользоваться услугами, оставьте заявку непосредственно на сайте.
Добро пожаловать на наш портал о недвижимости https://stroyengr.ru/ где вы сможете найти множество полезных статей на такие темы, как покупка квартиры в новостройке, приемка квартиры от застройщика, покупка и продажа недвижимости.
недорогие квартиры от застройщика купить 2 квартиру
https://rusakita.unoforum.pro/?1-18-0-00002627-000-0-0-1717609978
Всем игроманам привет!
В последние годы онлайн-казино завоевывают все большую популярность, и среди них особое место занимает jv spin casino. Это казино предлагает широкий спектр игр, щедрые бонусы и удобный интерфейс, что делает его идеальным выбором для новичков и опытных игроков.
Одним из главных преимуществ jvspin мобильная версия является его официальный сайт, который обеспечивает быстрый доступ ко всем функциям казино. Если вас как и меня интересует casino jvspin, jvspin casino бонус или казино дживиспин онлайн, то самое время прочитать здесь [url=https://beauty-experts.ru/2024/05/29/Игры-в-казино-без-депозита-всё-что-нужно-знать]beauty-experts.ru[/url] подробнее об jvspin казино регистрация. На сайте вы найдете актуальную информацию о новых играх, промоакциях и специальных предложениях, которые помогут увеличить ваши шансы на выигрыш.
Для тех, кто предпочитает играть на мобильных устройствах, jv спин предлагает мобильную версию, которая позволяет наслаждаться любимыми играми в любое время и в любом месте. Скачивание приложения проходит быстро и легко, а интерфейс адаптирован под разные платформы, что делает игровой процесс максимально комфортным.
Если вы хотите узнать больше о возможностях jvspin казино официальный, советуем обратить внимание на разделы, посвященные регистрации и входу в личный кабинет. Здесь вы найдете пошаговые инструкции, которые помогут быстро начать игру и воспользоваться всеми доступными бонусами и привилегиями.
Не забывайте следить за обновлениями и рабочими зеркалами казино дживиспин, чтобы всегда иметь доступ к своему аккаунту и любимым играм. Если вас как и меня интересует jv spin, jvspin казино официальный сайт или jvspin зеркало, то самое время прочитать здесь [url=https://nordu.ru/2024/05/29/Игровые-автоматы-без-депозита-лучшие-предложения]nordu.ru[/url] подробнее об дживиспин регистрация.
Играйте в удовольствие и помните: в jvspin casino бонусы каждый может стать победителем!
Более подробно на https://t.me/s/jvspin_zerkalo
Удачи и профитов!
jvspin зеркало рабочее
jv spin
дживи спин казино
jvspin сайт
jvspin рабочее зеркало
Привет любители азарта!
Если вас как и меня интересует игорный клуб лев зеркало, лев казино бездепозитный бонус или lev casino официальный, то самое время прочитать здесь [url=https://gps-fake.com/casino-game-devotion_859716.html]gps-fake.com[/url] подробнее об казино лев дополнительные выплаты от 1500 до.
Более подробно на https://t.me/s/playlevcasino
Удачи и профитов!
игровой клуб лев казино
lev casino регистрация
вулкан лев казино
лев казино бездепозитный бонус 30 fs
промокод казино лев деньги
На сайте https://cliningkrd.ru закажите звонок для того, чтобы воспользоваться услугами клинингового агентства. В компании работают компетентные и квалифицированные сотрудники, которые прошли необходимую подготовку. Используется инновационное и качественное оборудование, а также уникальные технологии, которые позволяют добиться блестящего результата. Бытовая химия полностью безопасна как для животных, так и людей, не провоцирует аллергии. Все услуги доступны по привлекательной стоимости. Они оказываются в минимальные сроки.
There’s nothing better than winding down after work with some adult entertainment. I found Mult34 – [url=https://mult34.com/category/boruto/]boruto porn[/url] , a site that offers a vast collection of erotic comics and cartoons for free. It’s become my favorite place to relax and enjoy quality content.
Mult34 – [url=https://mult34.com/liane-cartman-and-chef/]liane cartman[/url] diverse range ensures you’ll never get bored. The site has something for every preference, and the best part is that it’s all free. If you need a way to relax after a long day, check out Mult34 – [url=https://mult34.com/]porncomix[/url] .
Ищите промоакции в Казахстане? Зайдите на сайт https://probonus.kz/ и вы можете узнать о промо акциях в Казахстане, какие проходят акции в магазинах вашего города и быть в курсе где проходят новые акции и скидки. Акции постоянно добавляются и обновляются, что даст возможность существенно сэкономить на покупках.
На сайте https://napolnye-konditsionery.ru/ есть возможность приобрести напольные кондиционеры. Они подходят не только для обустройства дома, но их устанавливают и в офис. Такие устройства отличаются огромным количеством преимуществ, что и делает их незаменимыми в быту. Напольные устройства наделены большим количеством важных функций, которые делают их максимально удобными в использовании и практичными. Есть устройства премиального уровня, а также бюджетные. Выбирайте кондиционеры, оснащенные такими полезными опциями, как: осушение воздуха, фильтрация и различные виды охлаждения.
На сайте https://ocims.ru/ закажите звонок для того, чтобы воспользоваться такой услугой, как строительство каркасных домов высокого качества. Они надежные, практичные, а самое главное, что простоят очень долго. Все работы происходят под ключ. Дома строятся по индивидуальному, а также типовому проекту, что позволит подобрать то, что соответствует предпочтениям, вкусам. На работы, материалы даются гарантии. Действуют привлекательные и доступные расценки. Также компания предлагает приятный презент по окончании проведения работ.
Rassilka.kz оказывает качественные услуги. Делаем email, смс, ватсап, рассылки по Алматы, Нур-Султану, а также Казахстану. Сотрудничаем с разными популярными компаниями. Наша главная задача – принести всем клиентам пользу. Действия нацелены на конечный результат. https://rassilka.kz – сайт, где вы узнаете, почему нас выбирают. Здесь у вас есть возможность уже сейчас посмотреть тарифы. У нас вы получите консультацию на бесплатной основе. Мы с удовольствием ответим на все ваши вопросы, свяжитесь с нами в любое время.
На сайте http://smartporog.ru посмотрите каталог, где находятся выпадающие умные пороги, которые вы сможете приобрести прямо сейчас. Это решение станет оптимальным вариантом, если установка стационарного порога невозможна либо нецелесообразна в определенном случае. Посмотрите видео с тем, как такие пороги идеально вписываются в помещение. Они смогут устранить преграду, образовавшуюся в полу, избавляют от мостиков холода, повышают звукоизоляцию, борются с посторонними запахами.
play slots for real money
During the current digital time, the domain of gambling entertainment has experienced a extraordinary evolution, with internet-based casinos rising as the new frontier of fun and excitement.
Included among the the greatest enthralling components as part of this lively domain are the consistently-favored online slot machines, welcoming customers to embark on a adventure of thrilling interactivity and the prospect to win monetary prizes.
Virtual slot games have become a embodiment of joy and expectation for users across the world, delivering an unparalleled level of accessibility and availability.
Through merely a multiple selections, you can immerse yourself in a brilliant assortment of casino settings, each painstakingly crafted to ignite your experiences and heighten your engagement of your seat.
One of the primary allures of participating in slots for tangible prizes via the internet is the chance to feel the thrill of conceivably substantial prizes. The excitement of watching the icons rotate, the icons connect, and the major payout entice can be authentically stimulating.
Digital wagering establishments have effortlessly embedded state-of-the-art solutions to offer a interactive sensation that is equally visually mesmerizing and profitable.
Beyond the appeal of conceivable payouts, digital reel-based offerings as well grant a extent of flexibility and control that is unsurpassed in the typical casino context. You can customize your bets to match your financial resources, tweaking your gameplay to identify the sweet spot that fits your personal preferences and comfort with uncertainty. This amount of personalization equips players to build their financial resources and enhance their satisfaction, entirely from the convenience of their private abodes.
CVzen es el mejor servicio https://cvzen.es/ de redacción de CV y orientación profesional para millones de personas que buscan empleo en todo el mundo. Nuestro equipo de expertos está comprometido con tu éxito y te apoyará a lo largo de tu trayectoria profesional, desde la redacción de tu CV profesional y tu carta de presentación con datos específicos del sector hasta la optimización de tu perfil de LinkedIn y la orientación profesional. Desbloquea nuevas oportunidades con un currículum que muestre tu potencial.
На сайте https://lh3.ru/ вы получите всю нужную, содержательную информацию про то, как правильно организовать домашний уют, приятную и комфортную атмосферу. Вся информация будет интересна тем, у кого есть семья, дети. Этот блог содержит необходимые материалы о повседневной жизни. Так вы узнаете о том, как обустроить квартиру, чтобы в ней было приятно, уютно и привлекательно, хотелось туда возвращаться после работы. Также ознакомьтесь и с последними записями. Например, про именные браслеты и как часто необходимо подстригать челку, если у вас есть каре.
На сайте https://filtry-dlya-ochistki.ru/ в большом ассортименте находятся фильтры для воды. Они помогут значительно повысить качество обычной воды, а также улучшить ее характеристики. Вы сможете подобрать именно то, что подходит по техническим характеристикам. Фильтры помогут улучшить вкус воды, сделают ее намного чище. Кроме того, срок службы бытовой техники будет продлен. Фильтры необходимы для того, чтобы удалить хлор, тяжелые металлы, а также микроорганизмы. Выбирая подходящий вариант, необходимо учесть количество уровней очистки и другие моменты.
When you own a boat you experience enjoyment and pleasure. But there is another side of this: the maintenance of the boat. Our job https://ntdmarinegroup.com/ is to take over this side and leave you with only the pleasure and enjoyment.
https://zagorie.mybb.ru/viewtopic.php?id=9390#p23260
Интернет-магазин «Аврора Агро Партс» заслуживает вашего внимания. Предлагаем привлекательные цены на автозапчасти ведущих брендов. Всегда рады общению с нашими клиентами, мы их уважаем. Вы можете смело рассчитывать на помощь компетентного специалиста. Он грамотно подберет то, что вам действительно нужно. https://aa-p.ru – сайт, где можно в любое удобное для вас время ознакомиться с условиями оплаты и доставки. Заказы выполняем максимально быстро. Открыты для взаимовыгодного сотрудничества и регулярно развиваемся, обращайтесь!
Добро пожаловать на наш портал о недвижимости https://stroyengr.ru/ где вы сможете найти множество полезных статей на такие темы, как покупка квартиры в новостройке, приемка квартиры от застройщика, покупка и продажа недвижимости.
На сайте https://ruskiseerial.net/ представлены интересные и захватывающие дух фильмы, которые порадуют своей актерской игрой, интересной постановкой и происходящими событиями. В каждом фильме играют талантливые актеры, которые отлично исполняют свою роль и помогают зрителям отвлечься от повседневной суеты. Представлены самые разные жанры, включая драмы, мелодрамы, комедии, детективы, военные, криминал и многое другое. Есть раздел с теми произведениями, которые уже скоро появятся на сайте.
https://damntroublemaker.com/
На сайте https://elekspb.ru закажите судовой кабель, судовую электротехнику с регистром и многое другое. Продажа осуществляется оптом. В компании трудятся проверенные, надежные специалисты, которые помогут подобрать нужное решение. Компания энергично и активно развивается на рынке, что позволяет своевременно реагировать на изменения. Предприятие специализируется на поставках электрооборудования, а также материалов для судостроения, судоремонта. Для того чтобы подобрать оптимальное решение, изучите технические характеристики.
кактус сан педро купить семена
[url=https://gates-of-olympus-1000.fun/]Gates of Olympus 1000 на реальные деньги[/url]
самые крупные клининговые компании россии
Всем привет!
Сегодня я расскажу, как получить промокод на фриспины в new retro casino. Кто не любит бесплатные вращения, которые могут привести к крупным выигрышам? В этом посте я поделюсь с вами лучшими способами найти и использовать промокоды, чтобы увеличить свои шансы на успех.
Если вас как и меня интересует new retro казино рабочее зеркало, нью ретро казино зеркало или retro casino регистрация, ознакомьтесь тут https://t.me/s/NewRetroCasino_promokod с ретро казино промокод на пополнение
[url=https://newcouponsalerts.biz/2024/05/29/online-casinos-a-thorough-guide]newcouponsalerts.biz[/url]
Удачи и профитов!
можно ли вывести бонус с ретро казино
ретро казино фриспины бездеп
new retro casino мобильная версия
new retro casino промокоды на фриспины
ретро казино незаходиь
работа вебкам моделью https://studio-milano.ru/
На сайте https://autodix.ru/ почитайте всю интересную, увлекательную и актуальную информацию, которая касается не только автомобилей, но и авторынка. Перед вами любопытные советы, ценные рекомендации, познавательные лайфхаки и многое другое, что поможет лучше сориентироваться во всем многообразии материала. Регулярно публикуется новый материал, который вызовет у вас интерес. Описаны техники правильного и безопасного подключения крокодила. Кроме того, у вас получится разобраться в модификациях и сериях автомобилей различных марок.
На сайте https://kupit-velosiped1.ru/ в огромном выборе представлены функциональные, качественные и практичные велосипеды, которые созданы для активного отдыха. Выбирать устройство необходимо, полагаясь на бюджет, материал рамы. В каталоге вы найдете шоссейные, горные, детские, складные велосипеды и другие модели, которые реализуются по привлекательной и доступной цене. На всю продукцию предоставляются гарантии. Все изделия отличаются привлекательным дизайном и безупречным качеством.
Друзья, хотите узнать секреты успеха в онлайн-казино и получить бонусы от Sykaaa Casino? Тогда читайте дальше!
Сегодня я расскажу вам, как найти актуальные зеркала Sykaaa Casino, пройти регистрацию и получить крутые бонусы. Кстати, для всех новых игроков действует специальное предложение – дополнительный эксклюзивный бездепозитный подарок в размере 30 фриспинов в слоте The Dog House Megaways за регистрацию. Просто используйте промокод REG30FS и наслаждайтесь игрой!
Начнем с того, что Sykaaa Casino предлагает выгодные бездепозитные бонусы для новичков. Это отличная возможность начать играть и выигрывать без вложений. Промокод REG30FS предоставит вам 30 фриспинов с отыгрышем х35. Используйте этот шанс, чтобы испытать удачу и выиграть по-крупному!
Регистрация в Sykaaa Casino очень проста. Перейдите на официальный сайт или актуальное зеркало Sykaaa Casino. Нажмите кнопку “Регистрация”. Заполните необходимые поля: имя, email, пароль и другие данные. Подтвердите регистрацию через email. Войдите в аккаунт и введите промокод REG30FS, чтобы получить 30 фриспинов.
Если основной сайт Sykaaa Casino заблокирован, не переживайте! Актуальные зеркала помогут вам обойти блокировки и продолжить игру. Следите за обновлениями на нашем блоге, чтобы всегда иметь под рукой рабочие адреса.
Многие игроки уже воспользовались бездепозитным бонусом и поделились своими впечатлениями. Реальные истории успеха подтверждают, что играть и выигрывать в Sykaaa Casino не только возможно, но и легко!
В Sykaaa Casino представлено множество игровых автоматов на любой вкус. Вы сможете найти популярные слоты, такие как The Dog House Megaways, и наслаждаться игрой в любое время и из любой точки мира.
Не упустите возможность получить 30 фриспинов в слоте The Dog House Megaways за регистрацию. Используйте промокод REG30FS и начинайте выигрывать уже сегодня! Преимущества Sykaaa Casino очевидны: выгодные бонусы, удобный доступ через зеркала и широкий выбор игр.
Если вы хотите узнать о СУКААА больше, ищите в поиске казино суккка сукко официальный или казино сукаа вход официальный сайт и вы сразу обнаружите нужную информацию или прочитайте о sykaaa зеркало сайта по ссылке https://t.me/csykaa
Удачи и больших выигрышей!
[url=https://yauveren.com/a-history-of-slot-machine-innovation_724465.html]yauveren.com[/url]
сукааа casino регистрация
sykaaa casino онлайн
sykaaa casino отзывы
скачать казино sykaaa
регистрация в сукааа казино
Посетите канал телеграм https://t.me/pinup_vhod_zerkalo_casino_sport и вы найдете Pin-Up Пинап Bonus & Promo | Pinup Пин Ап Регистрация, вход, скачать, зеркало | Casino | Link Kirish Giris | Mobile App. Сайт Pin-Up Пинап Россия, Казахстан, Узбекистан, Turkey
Pinup Пинап Букмекер Казино.
Internet trading https://www.5692.com.ua/ru/news/3780111/gde-obucitsa-finansovoj-gramotnosti-kak-ne-poterat-a-priumnozit-sredstva is the ability to benefit from fluctuations in the rates of market assets (stocks, currencies, gold, oil, etc.). Most wealthy people in the world are fluent in this skill, and this is one of their sources of income even during a crisis. After all, you must admit that no matter what happens in the economy, there will always be something that gets more expensive and something that gets cheaper. And if you understand market patterns and know how to analyze trends, then you can certainly make money from it.
На сайте https://ost-profi.ru/ закажите качественные, надежные, функциональные и эстетически привлекательные перегородки, которые созданы для зонирования помещения. Компания предлагает приобрести стеклянные ограждения, которые используются в душе, мобильные перегородки, а также цельностеклянные. Все офисные перегородки реализуются по доступной и привлекательной стоимости. Есть возможность создать варианты абсолютно любых размеров. Возможно, вам понравятся те перегородки, которые созданы в лофт направлении.
Канал [url=https://t.me/s/novuy_zaim_sankt_peterbeug]Новые займы в СПБ[/url] предлагает подборку самых новых займов на карту онлайн. Здесь вы найдете предложения от лучших МФО, готовых выдать займ каждому от 18 лет, независимо от кредитной истории и занятости. Получите до 30 000 рублей на выгодных условиях, первый займ под 0%. Узнайте больше о новых займах и выбирайте лучшее предложение для себя. Подписывайтесь на наш канал и будьте в курсе всех новинок.
Зайдите на сайт https://came.name/ где вы сможете ознакомиться с широким ассортиментом оборудования CAME (итальянский производитель), таким как автоматика для ворот, шлагбаумы, парковочные системы, турникеты и многим другим. Узнайте больше информации о продукции на сайте, а также посмотрите прайс листы с выгодными ценами.
На сайте https://biancorosso.ru/ ознакомьтесь с увлекательной, интересной и актуальной информацией на самую разную тему. Так вы узнаете о том, какой цвет волос подойдет именно вам, особенности стрижки каре на длинные волосы. Присутствуют и любопытные, содержательные уроки макияжа. Вы научитесь правильно наносить косметику, чтобы в любой ситуации выглядеть очаровательно. Кроме того, имеется информация относительно того, какой макияж актуален в этом году. Все рекомендации от стилистов и профессионалов, а потому на них точно можно полагаться.
На сайте https://margoritka.ru ознакомьтесь с полезной, интересной и содержательной информацией, которая посвящена здоровью, красоте. Так вы узнаете о том, как справиться с сухостью кожи, о причинах гипотиреоза у женского населения и как с этим недугом справиться. Также вы ознакомитесь с особенностями аппарата, предназначенного для промывания носа. Имеется информация и о шариках Кегеля. Вы узнаете о том, как решить проблему, связанную с сухостью в интимной зоне. Также есть данные и о зубных протезах.
Безупречный вид вашего авто: детейлинг в столице.
Детейлинг Москва [url=https://www.detailing-uslugi.ru/]https://www.detailing-uslugi.ru/[/url] .
Какие услуги предлагаются при перетяжке салона автомобиля в столице?
Перешив салона автомобиля цена Москва [url=http://www.poshiv-avtosalona.ru/]http://www.poshiv-avtosalona.ru/[/url] .
На сайте https://vpolshe.com/pesel-bez-prisutstviya/ вы сможете заказать оформление песеля в Польше. Для того чтобы воспользоваться услугой, необходимо заказать специальную исчерпывающую консультацию, на которой менеджер расскажет, что необходимо для оформления важного документа. Здесь же можно сделать песель и для малыша. Стоимость указана на сайте, а срок изготовления – не более 3 дней. Также возможно восстановление документа, если тот был утерян в Варшаве. Вам вышлют скан в формате PDF. При необходимости будет получен документ на сотрудников.
На сайте https://stekloholst-77.ru/ в огромном ассортименте представлены стеклохолсты. Они представляют собой такой обойный материал, который производится из стекловолокна. Зачастую применяется с той целью, чтобы выровнять потолки, стены, а также сделать интерьер более привлекательным и красивым. Все изделия отличаются высоким качеством. Полотно наделено высокой прочностью, надежностью, а также экологической безопасностью. Нанесение стеклохолста не вызывает неудобств. А при выборе такого материала необходимо ориентироваться на плотность, а также размер рулона.
На сайте https://ntmsp.ru/ закажите звонок для того, чтобы приобрести продукцию компании ООО “НОВЫЕ ТЕХНОЛОГИИ И МАТЕРИАЛЫ”. Это развивающееся предприятие, которое работает в области создания комплектующих, которые применяются в радиотехнической промышленности. В каталоге вы найдете крепеж, ферритовые изделия, медицинские иглы, а также оснастку. В компании трудятся компетентные и опытные специалисты, которые предпринимают все, чтобы вы получили такую продукцию, которая соответствует самым высоким требованиям.
Pro88
Pro88
Натяжные потолки в Москве https://potolki-pk.ru/ от профессионалов! Бесплатный выезд замерщика. Натяжные потолки под ключ, все возможные варианты, от базовых проектов, до сложных решений. Установка, монтаж, собственное производство! Подробнее на сайте.
На сайте https://mammas.ru/ почитайте увлекательную информацию на самую разную тему. Так есть статьи на тему психологии, красоты, отдыха, здоровья, детей, моды, дома, а также питания. Также почитайте публикации, касающиеся того, что сейчас находится в тренде и является актуальным. Имеются данные о том, кто такой мужчина-нарцисс, как его правильно распознать, кому подходит каре с челкой и какие варианты стрижки существуют. Есть информация на тему личных границ, как их сохранить и сберечь. Кроме того, вы будете в курсе того, чем лучше заняться в летнее время. Перед вами самые эффективные идеи.
На сайте https://polvteplo.ru/ в огромном ассортименте представлены нагревательные маты, кабели, инфракрасный пол, а также терморегулятор, который используется для теплого пола и многое другое. Есть вариант подобрать все необходимое по типу напольного покрытия: под ламинат, плитку, керамогранит, паркетную доску. Здесь вы сможете не только приобрести все компоненты для теплых полов, но и заказать поставку, проектирование, а также монтажные работы. Для того чтобы лучше разбираться в теме, изучите полезные информационные статьи.
Компания https://primeinterior.ru/ занимается поставками натурального мрамора, гранита, травертина, оникса и кварцита со всего мира для премиальных интерьеров. Имеем собственное производство во Владивостоке и команду специалистов для монтажа. Готовы к крупным заказам любой сложности со всей России, есть опыт работы с отелями, ресторанами, ночными клубами. Создаём неповторимые интерьеры из природного камня, высокое качество работы и материалов.
На сайте https://sulyaev.ru/ изучите информацию о практикующем и профессиональном психологе-психоаналитике Андрее Суляеве. Он поможет решить даже самые сложные, запутанные дела, которые требуют особенного внимания к деталям. Поэтому если и у вас накопились проблемы, то воспользуйтесь помощью этого специалиста. Основная деятельность психолога заключается в частных психологических практиках. При этом установлены привлекательные расценки на услуги. Он с радостью ответит на все вопросы и даст советы.
На сайте https://potolochnye-svetilniki.ru/ в огромном многообразии представлены потолочные светильники, которые добавят уюта, сделают ваш дом более комфортным, интересным и привлекательным. Многие выбирают потолочные светильники по той причине, что они отличаются высоким качеством, реализуются по доступной стоимости. Специально для вас безопасная доставка. Прямо сейчас вы сможете приобрести светильники для любого типа помещения, включая кухню, спальню, коридор, зал. Для ванной получится подобрать водонепроницаемые решения.
На сайте https://nabor-curierov.ru/ozon/ оставьте заявку для того, чтобы стать курьером ОЗОН и получать регулярные заказы. Если вы будете развозить заказы на личном автомобиле, то сможете получить 5 500 р. в день, а если на авто компании, то 7 000. Вы устраиваетесь на работу как самозанятый. Вас ожидает стабильная работа, а выплаты происходят каждую неделю, что очень удобно. А если выполните брендирование авто, то получите сверху еще 5 000 рублей. Если возникла непредвиденная ситуация, то всегда сможете рассчитывать на оперативное решение вопроса.
На сайте https://advokat-samara.ru/ вы сможете воспользоваться услугами квалифицированного, опытного адвоката по уголовным делам Мотина Анатолия Владимировича. У него профильное высшее образование, а в юриспруденции находится с 2002 года. Он на профессиональном уровне решает такие дела, как: ДТП, происшествия на воде, авиакатастрофы, транспортные преступления. В том случае, если вы находитесь в поисках лучшего и опытного специалиста, то воспользуйтесь услугами данного юриста.
промокоды все инструменты
На сайте https://vpolshe.com/propiska-v-polshe-g-varshava-dogovor-arendy-zhilya-varshava/ вы сможете оформить такой важный документ, как прописка в Польше, Варшаве. Сотрудники компании оформят договор аренды жилья. Они проведут исчерпывающую, содержательную консультацию, на которой вы узнаете всю необходимую информацию, в том числе, и то, какие документы вам потребуются для оформления. Для наглядности присутствует и образец. Прописка потребуется для открытия счета, оформления карты побыту.
https://bi-cleaning.ru/
https://mclean.su/
На сайте https://gotovi.ru/ ознакомьтесь с интересной, увлекательной информацией о том, что необходимо современной женщине. Есть материалы об аксессуарах, как и с чем лучше сочетать сумки определенного цвета и материала. Вы узнаете, как всегда оставаться на стиле, быть модной, но при этом не переплачивать за бренды. Есть информация о том, что должно быть в гардеробе как на каждый день, так и для дома. Имеются публикации на тему туризма – как правильно собирать чемоданы и подготовиться к поездке.
Главных преимуществ кварцвинилового ламината является его высокая стойкость к истиранию, царапинам и влаге. Это делает его идеальным для использования в кухне, ванной комнате, прихожей и других помещениях с высокой проходимостью. Кварцвинил легко чистится и устойчив к пятнам, что делает его идеальным для семей с детьми и домашними животными. Виниловые полы
aviator online game [url=https://aviator-crash-game.ru/]https://aviator-crash-game.ru/[/url] .
Зайдите на сайт АКСЕЛЬ https://axsel-murmansk.ru/ где вы сможете купить новый автомобиль или автомобиль с пробегом в Мурманске. Ознакомьтесь с каталогом авто в наличии, выберите по параметрам и стоимости. Также работает быстрый выездной выкуп по самым выгодным ценам. Предоставляем услуги кредитования, лизинга, страхования.
CVzen https://cvzen.it/ è il servizio di scrittura di CV e career coach per eccellenza per milioni di persone in cerca di lavoro in tutto il mondo. Il nostro team di esperti si impegna per il vostro successo e vi supporterà in tutto il vostro percorso professionale, dalla stesura di un CV professionale e di una lettera di presentazione specifica per il settore, all’ottimizzazione del vostro profilo LinkedIn, fino al career coaching professionale. Sbloccate nuove opportunità con un curriculum che metta in luce il vostro potenziale.
MIGRANT SUPPORT предлагает услуги квалифицированных специалистов, которые имеют большой опыт работы. В своей деятельности мы каждому клиенту предоставляем индивидуальный подход. Гарантируем достижение в выгодном совместном сотрудничестве и приемлемые цены. https://vpolshe.com/kriptolitsenziya-v-polshe/ – сайт, где у вас есть возможность уже сегодня оставить заявку. Поможем вам получить криптолицензию в Польше. Процесс пройдет хорошо, мы все выполним оперативно. Вы останетесь довольны результатом и сервисом. Обращайтесь к нам, на все вопросы мы ответим с большим удовольствием!
На сайте https://premier-pokrytie.ru/ есть возможность заказать резиновое покрытие, которое используется внутри помещения, а также на детских, спортивных площадках. В компании трудятся компетентные и лучшие специалисты, у которых огромный опыт работы в данной сфере. Они в совершенстве знакомы с технологией создания резинового покрытия, что позволяет предложить продукт безупречного качества и с уникальными техническими характеристиками. Специалист приедет к вам на объект для замеров абсолютно бесплатно.
На сайте https://omsksite.ru/ представлены самые эффективные, работающие методы выполнения ремонта. Этот блог посвящен строительным, ремонтным, отделочным работам. Вся информация является полезной, интересной. А самое главное, что позволит справиться с проблемой максимально быстро. На сайте будут рассказаны все тонкости ремонта и то, какие материалы для этих целей лучше использовать. Имеется контент на тему того, как правильно подобрать пластиковое окно. Есть информация про пожарную охрану, руководство, которое поможет правильно подобрать набор инструментов.
купить аттестат школы https://6landik-diploms.com
На сайте https://elektrosamokat1.ru вы сможете ознакомиться со всеми преимуществами, особенностями электросамокатов, которые считаются удобным и комфортным способом передвижения. Перед вами огромный выбор самых интересных и функциональных моделей. Они идеально подходят для пассажиров самых разных возрастов. Эти устройства представлены только от лучших производителей, которые положительно себя показали. Имеются варианты премиального уровня, а также более бюджетные. Этот вид транспорта не только экологически чистый, но и экономичный, но при этом достаточно мощный.
На сайте https://www.okna-mechtipro.ru/ закажите двери, окна, которые производятся из ПВХ. Здесь практикуются на создании ПВХ-конструкций самого разного предназначения и вида. Прямо сейчас закажите бесплатный замер. На все работы, конструкции дается гарантия – 3 года. Доставка, монтажные работы осуществляются за один день. Компания всегда устраивает для своих клиентов выгодные акции, делает скидки. Окна точно прослужат очень долго! Есть возможность заказать окна самых разных цветов и форм. Продажа качественных конструкций сопровождается сервисом высокого уровня.
На сайте https://ru.cryptolegalservices.io/ вы сможете воспользоваться такими полезными услугами, как: оформление крипто-лицензии, нормативный анализ, аудит. Вы сможете воспользоваться бухгалтерскими, налоговыми услугами для тех компаний, которые работают с токенами. Также можно приобрести и недвижимость за токены. Предприятие обслуживает частных лиц, а также различные криптовалютные биржи. Специалисты работают таким образом, чтобы вы смогли работать максимально безопасно и с комфортом.
рассчитать такси https://taxi-novocherkassk.ru/
На сайте https://t.me/s/m_1go изучите всю необходимую информацию, которая касается онлайн-заведения 1GO. Воспользуйтесь возможностью сыграть и выиграть приличную сумму денег. Официальный канал находится в вашем мобильном телефоне. Почитать всю интересующую информацию получится в любое время, даже поздним вечером. Открывайте группу на своем мобильном телефоне либо компьютере, чтобы получить доступ к безграничному миру развлечений, бонусов, привилегий. Теперь и вы сможете присоединиться к обществу победителей!
Ищете способ расслабиться и получить незабываемые впечатления? Мы https://t.me/intim_tmn72 предлагаем эксклюзивные встречи с привлекательными и профессиональными компаньонками. Конфиденциальность, комфорт и безопасность гарантированы. Позвольте себе наслаждение и отдых в приятной компании.
На сайте https://top-volna.ru воспользуйтесь уникальной возможностью послушать любимую радиостанцию и просто получить отменное настроение от приятно проведенного времени. На этом портале такие радиостанции, как: Маруся ФМ, Русское Радио, Новое Радио, Европа Плюс, DFM, Радио Энерджи, Ретро FM, Юмор FM и многое другое. Прямо сейчас послушайте самые популярные песни, которые нравятся многим. Вас покорит отличное звучание, а также интересная аранжировка, которая точно никого не оставит без внимания.
сколько стоит такси в новочеркасске https://zakaz-taxionline.ru/
IFran.ru – это онлайн-проект, созданный для повышения искренности и прозрачности рынка франчайзинга в РФ. Портал собирает и анализирует объективные данные из разных открытых источников, а затем представляет полученную информацию в структурированной и единообразной форме. https://www.ifran.ru – сайт, где у вас есть возможность узнать о том, что из себя представляет франчайзинг. Здесь имеются интересные статьи, обзоры и рейтинги. При помощи возможностей нашего сервиса потенциальные франчайзи могут осуществить более обдуманный выбор в пользу качественных франшизеров.
Займ с любой кредитной историей. Без подтверждения дохода. Вы остаётесь собственником. Без отказов. Быстрое оформление. Без скрытых комиссий [url=http://matkapital38.ru/]займ на ип[/url]
https://faina-cleaning.ru/
На сайте https://gazelka86.ru/ закажите грузоперевозки с хорошей скидкой до 50%. Это очень удобно, потому как в компании работают первоклассные, компетентные специалисты, которые выполнят все работы на высоком уровне, профессионально и качественно. Под груз выделяется свой транспорт, который подбирается в соответствии с габаритами, объемом, весом. Предоставляется полный комплекс услуг, чтобы вы воспользовались именно тем, что нужно. Учитывая небольшую стоимость, дается еще и скидка.
Дорамы стали настоящим культурным феноменом, покорившим сердца зрителей по всему миру. Если вы хотите окунуться в мир азиатских сериалов, посетите сайт [url=https://doramaserials.net/]doramaserials.net[/url]. Здесь можно дорамы смотреть онлайн в высоком качестве. Удобный поиск и обширная библиотека помогут вам быстро найти нужный сериал. Погружайтесь в увлекательные истории и наслаждайтесь просмотром без ограничений.
На doramaserials.net вы можете найти [url=https://doramaserials.net/]дорамы бесплатно[/url]. Это отличный способ наслаждаться любимыми сериалами без лишних затрат. Сайт предлагает широкий выбор дорам для любого вкуса. Откройте для себя мир азиатского кинематографа и погрузитесь в атмосферу восточной романтики и драмы. Смотрите дорамы с удовольствием и открывайте новые горизонты!
http://www.russa24-diploms-srednee.com
Зайдите на сайт https://came.name/ где вы сможете ознакомиться с широким ассортиментом оборудования CAME (итальянский производитель), таким как автоматика для ворот, шлагбаумы, парковочные системы, турникеты и многим другим. Узнайте больше информации о продукции на сайте, а также посмотрите прайс листы с выгодными ценами.
купить медицинскую справку
На сайте https://avangardmet.ru вы сможете воспользоваться такими нужными услугами, как: проектирование, обратный инжиниринг, механическая обработка и многое другое. Предприятие ООО “Авангард” успешно развивается, чтобы предложить потребителю только лучшее и прогрессивное. Оно производит, поставляет детали для компаний, связанных с машиностроением, авиационной, медицинской промышленностью. Важной задачей является производство аналогов импортных деталей эталонного качества. Инженеры отличаются высшим образованием.
Официальный дилер Москвич в Мурманске https://axsel-moskvich.ru/ с автомобилями в наличии. Зайдите и ознакомьтесь с модельным рядом и ценами, спец предложениями и акциями. Воспользуйтесь конфигуратором автомобиля, а также действующими выгодными кредитными программами или запишитесь на ТО.
Accepted by all major bookmakers, casinos and poker rooms, your Skrill wallet lets you enjoy online betting and gambling without revealing your personal payment [url=https://cz-kasina.cz/casino/beep-beep-casino/]visa online kasina[/url]
vong lo?i euro 2024
На сайте https://ipoteka.ru/ вы сможете воспользоваться такой услугой, как помощь в оформлении ипотеки, сопровождение различных сделок, которые совершаются с недвижимостью. В компании работают лучшие, компетентные сотрудники, которые дадут консультацию, а также на профессиональном уровне подберут оптимальную программу для оформления ипотеки. Сервис практикует сотрудничество с ведущими банками, а также инвестиционными предприятиями России, а потому есть возможность получить льготу по ипотеке. Только для вас самые лучшие предложения, которыми воспользуйтесь прямо сейчас.
На сайте https://t.me/s/gatesofolympus_1win вы сможете получить всю необходимую официальную информацию, которая касается канала «Gates of Olympus». Здесь самая актуальная, правдивая информация, которая вам потребуется, если вам нравятся азартные развлечения. Многие пользователи отмечают то, что у компании высокая вероятность получения выигрыша. Перед вами огромное количество интересных, увлекательных слотов, развлечений, которыми вы сможете воспользоваться прямо сейчас.
На сайте https://tursmir.store/ почитайте любопытную, содержательную информацию о том, как правильно отдыхать летом с пользой для организма и своего кошелька. Здесь же вы найдете и любопытные новости, которые касаются отдыха в гостиницах Сочи. Вы узнаете о том, какие нюансы таит в себе принятие солнечных ванн, есть ли вред от этого. Авторы публикуют и различные меры предосторожности, чтобы вы загорали правильно. Получите информацию о том, как забронировать отель. Возможно, вас заинтересуют различные гастрономические туры.
На сайте https://vavada1v.fun/ пройдите регистрацию для того, чтобы начать играть в казино Вавада. Оно обрадует вас огромным количеством удивительных, разнообразных развлечений, бонусами. А самое главное, что заслуженный выигрыш вы получите сразу же, без задержек. После регистрации для вас открывается удивительный мир ярких эмоций и безграничного количества развлечений, а потому вы точно будете знать, чем заняться сегодня вечером. Заходить сюда можно, в том числе, с мобильного телефона и любого другого гаджета.
Best online casinos in 2024 reviewed, tested, and ranked by gambling experts. Expert Online Casino Reviews independently review & rank each online casino with our 25 step process [url=https://vlcngrand.com/casino/marvel-casino/]pelican casino register[/url]
Техническое заключение о перепланировке помещения alma-stroi.ru
Перепланировка помещения — один из самых главных этапов в строительстве различных объектов. Но в нашей стране, она непременно должна быть узаконена и выполненной по всем правилам. Мы знаем о перепланировках всё, изучите на сайте alma-stroi.ru уже сейчас.
По запросу [url=https://alma-stroi.ru/]сколько стоит согласование перепланировки[/url] мы окажем помощь Вам. Если у Вас уже проведена несанкционированная перепланировка без документов, то это не беда. Её просто можно узаконить и спокойно пользоваться зданием. Не всегда расположение комнат в доме или промышленных помещениях устраивает хозяина. Но в крайнее время, перепланировка просто лучший выход из ситуации. Конечно, выгоднее всего ее проводить на этапе начального ремонта, но если этого не произошло, то её можно сделать на каждом этапе пользования.
Прайс на перепланировки можно посмотреть на интернет сайте alma-stroi.ru или посмотреть примеры готовых работ. Мы работаем в представленной теме уже множество лет и имеем много счастливых клиентов и готовых проектов. К любому проекту имеем персональный подход и учитываем все пожелания заказчика. Также работаем четко в установленный срок и по очень выгодным расценкам.
Оформить заказ на [url=https://alma-stroi.ru/]разработка проекта перепланировки[/url] можно уже сейчас. Наши специалисты приедут к Вам для измерения помещений и выявления размера работы. И после этого будет названа окончательная цена и день выполнения работ. Перепланировка — это прекрасная возможность сделать свою жизнь комфортнее.
https://vk.com/kent_bonus
Любите корейские дорамы и хотите наслаждаться ими без рекламы и в хорошем качестве? Сайт [url=https://doramaserials.net/doramy-korejskie/]doramaserials.net[/url] предлагает вам уникальную возможность смотреть корейские дорамы онлайн в любое время и в любом месте. Здесь вы найдете огромный выбор сериалов, который удовлетворит даже самых взыскательных зрителей. Удобная навигация по сайту и регулярные обновления каталога помогут вам всегда быть в курсе последних новинок.
Смотреть [url=https://doramaserials.net/doramy-korejskie/]корейские дорамы онлайн[/url] на doramaserials.net – это наслаждение без отвлекающих факторов. Высокое качество изображения и звука делают просмотр еще более захватывающим. Погружайтесь в мир корейских дорам, где каждый сериал – это настоящая находка. Откройте для себя волшебство азиатского кинематографа на нашем сайте и наслаждайтесь любимыми шоу без ограничений!
На сайте https://seobomba.ru/ закажите такую полезную и важную услугу, как продвижение портала вечными ссылками. Такая раскрутка считается одной из самых востребованных в этом году. А для того, чтобы сэкономить финансы, лучше заказать услуги напрямую, без посредничества. Все ссылки проходят детальный отбор, чтобы предложить продукцию эталонного качества. Предлагается гибкая система расценок. Гарантируется прозрачность работ и исключительно с лучшими партнерами, которым точно можно доверять.
На сайте http://www.tribal-tattoo.ru ознакомьтесь с работами, которые создают искусные, талантливые мастера тату-салона «Tribal». Они сделают тату в самых разных направлениях и независимо от сложности. Все самые интересные, неповторимые и креативные идеи будут в обязательном порядке реализованы. В работе используются только качественные и одноразовые расходные материалы, высокоточное оборудование, которое позволит получить нужный результат. Кроме того, выполняется перекрытие тех работ, которые были исполнены некачественно. При помощи тату получится скорректировать шрамы и рубцы.
Техническое заключение о перепланировке помещения alma-stroi.ru
Перепланировка квартиры — один из самых главных этапов в ремонте различных объектов. Но в нашей стране, она непременно должна быть законной и выполненной по всем нормам. Мы представляем о перепланировках всё, смотрите на сайте alma-stroi.ru уже сейчас.
По вопросу [url=https://alma-stroi.ru/]согласование перепланировки зданий в москве[/url] мы поможем Вам. Если у Вас уже выполнена самовольная перепланировка без документов, то это не беда. Её точно так же возможно узаконить и с легкостью пользоваться помещениями. Не всегда расстановка комнат в доме или промышленных помещениях устраивает владельца. Но в последнее время, перепланировка просто лучший выход из сложившейся ситуации. Безусловно, правильнее всего ее осуществлять на этапе чернового ремонта, но если этого не произошло, то её можно сделать на любом этапе эксплуатирования.
Цена перепланировки можно увидеть на онлайн портале alma-stroi.ru или увидеть примеры выполненных работ. Мы осуществляем работу в представленной теме уже большое количество лет и имеем множество довольных клиентов и выполненных проектов. К любому проекту имеем индивидуальный подход и учитываем все желания клиента. Также работаем четко в установленный срок и по очень приемлемым расценкам.
Оформить заказ на [url=https://alma-stroi.ru/]как узаконить перепланировку[/url] можно прямо сейчас. Наши специалисты приедут к Вам для замера комнат и выявления размера работы. И после этого будет посчитана окончательная цена и день выполнения работ. Перепланировка — это отличная вероятность сделать свою жизнь удобнее.
смотреть атака титанов в хорошем качестве https://ataka-titanov-anime.ru
На сайте https://geocarshield.ru у вас получится оформить страховку в Грузию. При этом все манипуляции выполняются в режиме реального времени. Вам не нужно долго стоять в очереди, ожидая своего момента. Оформление происходит всего за 5 минут. Вы сможете получить полис на автомобиль всего в несколько шагов. Оплата происходит любым, наиболее удобным для вас способом! Если появились какие-то вопросы, то вы сможете получить ответы на них, ведь служба поддержки работает круглосуточно! Прямо сейчас оформите страховку на легковой автомобиль, мотоцикл, автобус, прицеп, спецтехнику.
https://b2b-point.io/orci-varius-natoque-penatibus/
Glory Casino BD
МПК Гермес – ведущий поставщик металлопроката в Красноярске, основанный в 2011 году, специализируется на продаже арматуры, труб, швеллеров, уголков, профлистов и листов. За годы работы мы стали лидерами по поставкам металла в Красноярском крае, обслуживая предприятия и частных лиц.
На сайте https://kladupechi.ru почитайте всю полезную и важную информацию про печное отопление. Есть данные про голландскую, русскую, финскую печи. На портале опубликованы свежие и содержательные записи на самую разную тему. Все материалы сопровождаются цветными, яркими картинками. Для наглядности изучите видеоматериалы, на которых раскрывается секрет того, как правильно и безошибочно выполнять работы. Имеется видео про каминопечь, мангал барбекю и многое другое. Есть данные про кладку банных печей.
syair hk hari ini
На сайте https://v-electronic.ru/ вы сможете приобрести электронику от самых разных производителей: телевизоры, различные гаджеты, смартфоны, технику для кухни, дома, фото, видео, все для укладки волос, включая плойки, фены, ирригаторы, массажеры и многое другое. Имеется раздел, в котором представлен товар дня. Перед вами самые интересные, выгодные предложения, а потому спешите быстрее совершить приятную покупку до подорожания. Доступна экспресс-доставка – уже через 2 часа курьер приедет с посылкой.
Идеальная коляска Cybex для вашего малыша, новинки.
Лучшие оферты на коляски Cybex, для истинных ценителей качества.
5 причин выбрать именно коляску Cybex для вашего малыша, которые заставят вас влюбиться в этот бренд.
Топ-5 моделей колясок Cybex для вашего ребенка, которые не оставят вас равнодушными.
Элегантные решения для вашего ребенка – коляски Cybex, учитывая все особенности и пожелания.
Как правильно подобрать коляску Cybex для вашей семьи, исходя из индивидуальных потребностей и предпочтений.
Почему коляски Cybex так популярны среди родителей, которые ценят комфорт и безопасность.
Топ-модели колясок Cybex на любой вкус и цвет, которые порадуют вас своим разнообразием и качеством.
5 важных критериев при выборе коляски Cybex, для вашего малыша.
Какая коляска Cybex лучше всего подойдет вашей семье?, чтобы сделать правильный выбор.
Элегантные решения для вашей семьи – коляски Cybex, которые не оставят вас равнодушными.
5 важных критериев при выборе коляски Cybex, которые порадуют вас своим качеством и функционалом.
Идеальная коляска Cybex: комфорт и удобство для вашего малыша, которые стоит рассмотреть перед покупкой.
Коляска Cybex для вашего малыша: лучшие модели, если вы цените качество и комфорт.
Выбор коляски Cybex для вашего малыша: как не ошибиться, которые ценят надежность и стиль.
Топ-модели колясок Cybex для вашей семьи, перед совершением покупки.
Как выбрать идеальную коляску Cybex для вашей семьи, исходя из личных предпочтений и потребностей.
Особенности выбора коляски Cybex: как сделать правильный выбор, которые не оставят вас равнодушными.
прогулочная коляска кубекс [url=https://kolyaskicybex.ru/]прогулочная коляска кубекс[/url] .
https://guardian.ng/news/del-mar-energy-new-horizons-of-development-in-the-sea-of-japan-region/
купить мебель каталог
https://formomebel.ru/stoliki/metallicheskie
купить диплом врача [url=http://www.russa24-diploms-srednee.com]http://www.russa24-diploms-srednee.com[/url] .
online play aviator game [url=https://aviator-crash-game.ru/]aviator-crash-game.ru[/url] .
aviator game tricks [url=https://aviator-crash-game.ru/]https://aviator-crash-game.ru/[/url] .
aviator game real money [url=http://www.aviator-crash-game.ru]aviator game real money[/url] .
what is the best online casino that pays real money online casino
Professioneel CV https://cvzen.nl/ om je droombaan te krijgen Onze carriere-experts, redacteuren en geavanceerde technologie zullen je helpen om je carriere naar een hoger niveau te tillen.
Зайдите на https://tgramcat.com/ где вы найдете каталог телеграмм каналов всевозможных тем и направлений. Каналы тг постоянно пополняются и обновляются, что позволит найти интересное для вас. Каталог тг каналов можно искать по названию или узнать статистику по ним. Телеграмм каналы разбиты по категориям, но можно посмотреть подборку лучших. Вы можете добавить и свой канал.
Hello to all residents of the site novodiax.com, I apologize for being “off topic”, but I really need help. You need an authoritative opinion. I order a website on the topic of spare parts and here I found a more or less website, they trade [url=https://39jcb.ru][color=#1C1C1C]JCB[/color][/url] spare parts, I think I’ll take it from him appearance. I really want to hear your opinions on how convenient and interesting it will be for people, and I will also listen to a proposal for making the same site and better. The payment will be decent.
[url=https://noguchi-cc.com/pages/3/b_id=7/r_id=1/fid=83723667e8bb8f5fabebf776873a23a5]JCB Spare Parts[/url] [url=https://www.notonlyspaghetti.com/hello-world/#comment-12]JCB Spare Parts[/url] [url=http://ninfoblog.gp114.net/bbs/board.php?bo_table=qa&wr_id=17708]JCB Spare Parts[/url] 2e1e145
Opened up an enthralling read – I’d like to share it with you https://floridei.ru/poleznosti/ant-models-sovershenstvo-v-eskort-uslugah-v-moskve.html
Перепланировка помещений в Москве alma-stroi.ru
Перепланировка здания — один из самых главных этапов в строительстве разных объектов. Но в нашей стране, она обязательно должна быть узаконена и исполнена по всем правилам. Мы представляем о перепланировках всё, читайте на сайте alma-stroi.ru прямо сейчас.
По запросу [url=https://alma-stroi.ru/]разрешение на перепланировку[/url] мы поможем Вам. Если у Вас уже выполнена самостоятельная перепланировка без документов, то это не страшно. Её точно так же возможно узаконить и спокойно пользоваться зданием. Не всегда расположение комнат в доме или производственных помещениях устраивает хозяина. Но в крайнее время, перепланировка просто лучший выход из ситуации. Безусловно, лучше всего ее делать на этапе начального ремонта, но если этого не произошло, то её возможно сделать на каждом этапе эксплуатирования.
Стоимость перепланировки можно увидеть на веб сайте alma-stroi.ru или увидеть примеры выполненных работ. Мы работаем в представленной области уже множество лет и имеем много счастливых клиентов и готовых работ. К любому проекту имеем индивидуальный подход и учитываем все пожелания заказчика. Также работаем строго в срок и по очень выгодным ценам.
Оформить заказ на [url=https://alma-stroi.ru/]согласование перепланировки зданий в москве[/url] можно уже сейчас. Наши работники приедут к Вам для измерения помещений и выявления объема работы. И после этого будет посчитана итоговая стоимость и дата выполнения работ. Перепланировка — это отличный шанс сделать свою жизнь удобнее.
Ищете удобный интернет-магазин электрооборудования? ЭлектроМастер – ваш надежный партнер, который предлагает только высококачественные товары. Мы принимаем оплату за покупки разными способами. Ищете стабилизаторы? Sprise.store – сайт, где вы можете посмотреть условия доставки и каталог. Стараемся со своими клиентами выстроить длительные отношения, мы их ценим. Если у вас возникли какие-либо вопросы, свяжитесь с нами по телефону, с удовольствием придем вам на помощь. Сэкономим ваше время и денежные средства. С нетерпением ждем ваших заказов!
http://medforum.5nx.ru/viewtopic.php?f=4&t=2567
врата олимпа [url=https://www.gates-of-olympus-ru.ru]врата олимпа[/url] .
купить диплом моториста [url=www.russa24-diploms-srednee.com/]www.russa24-diploms-srednee.com/[/url] .
голяк смотреть онлайн качестве голяк смотреть онлайн
какие препараты снимают интоксикацию https://lechenie-alkogolizma.kz/
Не знаете как принимать платежи в криптовалюте онлайн на вашем сайте? Зайдите на https://cryptocloud.plus/ и вы сможете быстро и бесплатно подключиться к нашим продуктам. Посетите сайт, узнайте о решениях и возможностях и всех наших преимуществах. Инструменты для удобной работы с криптовалютой к вашим услугам.
голяк смотреть в хорошем качестве сериал голяк
Аренда квартиры не дает права на длительное пребывание на Кипре. Но договор нужен, чтобы подать документы на визу и сделать медицинскую страховку [url=https://cyprusrent.ru/]кипр аренда жилья на длительный срок[/url]
娛樂城官網
娛樂城官網
https://promokod-tennisi.ru/
Сайт https://ru.pocket-option.online/ это возможность торговать бинарными опционами и широкий спектр мировых торговых активов. Посетите сайт, узнайте все преимущества – гибкая торговля, всестороннее обучение, разнообразные торговые инструменты, демо-счет, удобное пополнение и вывод средств, индикаторы и сигналы. Начать торговлю можно в 1 клик. Подробнее на сайте.
На сайте https://telegra.ph/Kak-kupit-firmu-s-oborotom-05-15 получите всю важную, ценную и свежую информацию, которая касается покупки предприятия с оборотом. Вы узнаете о том, как приобрести бухгалтерскую компанию. Предоставляется подробная инструкция. Представлена информация, которая касается того, как реализовать ООО без долгов. Имеются и пошаговые инструкции, как приобрести бухгалтерскую компанию и реализовать ООО, если есть один учредитель без долгов. Также есть информация и о том, как правильней реализовать компанию без долгов.
Выбор элитных колясок Tutis, выбор мамочек в-the-know, Лучшие цветовые решения от Tutis, Инструкция по эксплуатации коляски Tutis, Необходимые аксессуары для комфортной прогулки с ребенком, Tutis: идеальный выбор для активных семей, Почему Tutis лучше конкурентов?, Секреты долговечности и красоты вашей коляски Tutis, Почему Tutis – выбор ответственных родителей, Секреты комфортной поездки с Tutis, секреты комфортного выезда, Как выбрать коляску Tutis, подходящую для вашего стиля жизни?, Почему Tutis – марка будущего, новинки на рынке детских товаров, профессиональное мнение, коляска, подчеркивающая ваш образ, преимущества использования коляски Tutis
детские коляски тутис [url=https://kolyaskatutis.ru/]детские коляски тутис[/url] .
Вас интересует качественная чистка дымоходов в СПб и Ленобласти? Вам поможет мастер трубочист-печник. В работе он использует исключительно современные технологии. Если вы обратитесь к нему, то получите по доступным ценам квалифицированные услуги. Ищете трубочист СПб? Трубочист78.рф – сайт, где можете узнать более подробную информацию. Трубочист приятный в общении, он быстро справится с задачей, оставив все в идеальном порядке. Мастер ответит на интересующие вопросы. Ваша печь-камин будет снова радовать вас уютом и теплом в доме.
В питомнике «go Mikheeva Elena» вы можете приобрести себе щенка, он будет вам преданным другом. Заводчик японских собак Елена Михеева гарантирует своевременную консультацию и поддержку на протяжении всей жизни малыша в любое время и по интересующим вопросам. Ее щенки и собаки растут строго на натуральной еде, так они получают нужные витамины и микроэлементы для крепкого здоровья. https://shibainu-japan.ru – сайт, где можете прямо сейчас посмотреть фото. Также здесь есть возможность забронировать щенка Сиба Ину. Он будет вам дарить позитивные эмоции и радость.
Ищете проверенных букмекеров в России? Мы отобрали букмекерские конторы, которые прекрасно относятся к своим игрокам. Предлагаем вам рейтинг и постоянно поддерживаем его в актуальном состоянии. Ищете отзывы о бк? Bettingsport.pro – портал, где представлена только интересная и полезная информация, посмотреть ее можно в любое удобное время. Наш сайт стал надежным помощником для активных пользователей ставок на спорт в России. Публикуем отзывы пользователей бк, советуем их изучить. Комментарии дадут возможность определиться с выбором для ставок.
На сайте https://ardis-hotel.ru/ забронируйте номер в популярном и уютном отеле «Ардис», который создан для приятного отдыха и изучения достопримечательностей. Прямо сейчас вы сможете забронировать номер на определенную дату. В отеле имеется развитая инфраструктура, а приветливый и дружелюбный персонал поможет с размещением. Все питание готовят исключительно из свежих, отборных продуктов. В меню есть все необходимое. Вас ожидают комфортные и большие номера. Здесь есть возможность отдохнуть с детьми самого разного возраста.
На сайте https://top10-hotel.ru/ представлено огромное количество отелей на самый взыскательный вкус. Они находятся в самых разных городах и странах, чтобы вы устроили себе незабываемое путешествие. Прямо сейчас у вас получится изучить все отели. Перед тем, как принять решение, ознакомьтесь со всем каталогом отелей. Воспользуйтесь специальным каталогом, который подберет решение по рейтингу, отзывам, звездности отеля. Также стоит учесть и параметры отеля, общий рейтинг и многое другое. Установлены самые разные цены.
Посетите сайт GuidesGame https://guidesgame.ru/ и вы найдете коды подарков и бонусов для игр с уникальными наградами для игр на iOS, Android и PC. Вы сможете также узнать гайды для игр и увидеть базу различных игр. Промокоды на игры, коды Roblox и многое другое у нас.
Hi all!
Turn Your Crypto into a Cash Machine with Lido’s 25% Monthly Returns! [url=https://crypto-airdrops.org]Crypto Earnings[/url]
The cryptocurrency world is filled with new projects that promise high returns and innovative solutions. However, Lido stands out with particularly attractive conditions. This project allows investors to stake their assets and earn up to 25% profit per month from their deposits. If you’re looking to grow your capital, investing in Lido could be a smart move. Lido is dedicated to ensuring a high level of reliability and transparency for its investors. All operations are conducted through smart contracts on the Ethereum blockchain, guaranteeing the security and integrity of the invested funds. Regular security audits further reassure users of Lido’s dependability. One of the main advantages of Lido is the high returns achievable through token staking. With potential yields reaching up to 25% per month, Lido is one of the most attractive projects in the cryptocurrency market. This high return rate allows investors to see substantial capital growth in a short period if they use this tool correctly. Lido is designed to be user-friendly, making it accessible even for beginners. Investors can easily join the platform, lock their tokens, and start earning profits within minutes. There is no need for a complex setup or technical skills; all you need is an Ethereum wallet and a bit of ETH for gas fees. Lido is also a project that is constantly evolving and expanding. It offers new opportunities and tools for its users, with the project team actively working on platform improvements and adding new features to meet user needs and stay at the forefront of the industry. Investing in Lido is not just a chance to make money but also an opportunity to be part of an innovative project that is changing the game in the cryptocurrency space. Start staking your tokens today with Lido and enjoy high returns.
For more information please visit https://crypto-airdrops.org
Wish you profit and prosperity)
Crypto Finance
Crypto Returns
Crypto Market Analysis
Crypto Opportunities
DeFi Ecosystem
Наша компания https://instock-dv.ru/ продаёт товары для дома, строительства, бытовую химию и парфюмерию и еще более 10000 наименований товаров оптом. Сотрудничаем с более чем 100 производителями различных брендов товаров. Мы занимаем лидирующие позиции среди оптовых B2B компаний Дальнего Востока и готовы выходить на новые рынки.
форум вебкам моделей и моделей OnlyFans! Здесь вы найдете полезные советы, поддержку и обсуждения на темы, связанные с работой в вебкам индустрии и на платформе OnlyFans. Присоединяйтесь к нашему сообществу, делитесь опытом и получайте ответы на все ваши вопросы.
https://forum.vipcamclub.ru/
Недвижимость в новостройке на Северном Кипре. Недвижимость в новостройке на Северном Кипре является одним из самых популярных вариантов [url=https://www.newton-apartment.ru/]кипр апартаменты на берегу моря[/url]
Здравствуйте!
Недавно у меня возникла потребность в получении среднего образования, но постоянная работа и обязанности постоянно отнимают время, и у меня нет возможности этим заниматься. К моему счастью, друзья посоветовали мне, что можно легально приобрести диплом о среднем профессиональном образовании (диплом техникума, колледжа) на бланках государственного образца, пройдя обучение по упрощенной программе!
В интернете полно предложений, но как избежать мошенничества и выбрать подходящую компанию? Это весьма важный вопрос, который требует ответственного подхода. Я лично проверил, что на http://russa24-diploms-srednee.com/ содержится множество советов о том, как избежать ошибок и не потерять деньги зря. Как проверить компанию по продаже дипломов, на что обратить внимание, и конечно, там можно заказать диплом “под ключ” без предварительной оплаты.
В отличие от других коммерческих организаций, наша цель — не просто заработать, а помочь нашим клиентам. Мы предоставляем бесплатные консультации без ограничений по количеству вопросов. Наши специалисты — проверенные временем профессионалы, поэтому обращение к нам – верное решение, ведь мы гарантируем качество, соблюдение сроков и разумные цены на дипломы о среднем образовании на заказ.
Удачи и хороших оценок!
купить диплом среднее специальное образование
куплю диплом о среднем медицинском образовании
купить диплом в новосибирске о среднем образовании
купить мед диплом среднего образования
диплом среднее образование купить диплом с занесением в реестр
купить диплом о среднем профессиональном образовании спб
диплом о среднем медицинском образовании купить
купить диплом о среднем образование в самаре
купить диплом о среднем медицинском образовании
реально купить диплом о среднем специальном образовании
CLAPS – интернет-магазин, который предлагает приобрести прибор DreamStalker Expert. Современный гаджет предназначен для проведения необычных экспериментов в осознанном сне, практик саморазвития и самосовершенствования, преодоления фобий и кошмарных снов. Ищете маска осознанных снов дримсталкер? Claps.me – сайт, где есть каталог, в нем содержатся характеристики, фотографии и отзывы покупателей. Здесь вы сможете узнать, что в комплект поставки прибора «DreamStalker Expert» входит.
ทดลองเล่นสล็อต pg
Slotเว็บตรง – เล่นได้ทุกอุปกรณ์อิเล็กทรอนิกส์ที่ใช้งานอินเทอร์เน็ต
ในสมัยนี้ การปั่นสล็อตมีความสะดวกมากยิ่งขึ้น คุณไม่ต้องเดินทางไปยังสถานที่เล่นคาสิโนที่ไหน ๆ เพียงแค่มีเครื่องมือที่ใช้งานได้เชื่อมต่อกับอินเทอร์เน็ต คุณก็มีโอกาสสนุกกับการหมุนสล็อตออนไลน์กับ PG Slot ได้จากทุกที่
การเสริมสร้างเทคโนโลยีของ PG Slot
ที่ PG Slot เราได้สร้างสรรค์โซลูชั่นสำหรับการบริการเกมคาสิโนออนไลน์เพื่อตอบสนองความพึงพอใจให้มากที่สุด คุณไม่ต้องการติดตั้งแอปพลิเคชันหรือนำมาใช้งานแอปพลิเคชันใด ๆ ให้เสียเวลาหรือกินพื้นที่ในอุปกรณ์ของคุณ การบริการเกมสล็อตออนไลน์ของเราดำเนินการผ่านเว็บตรงที่ใช้เทคโนโลยี HTML 5 ที่ล่าสุด
หมุนสล็อตได้ทุกอุปกรณ์
คุณอาจเล่นสล็อตออนไลน์กับ PG Slot ได้อย่างสะดวกเพียงเข้าถึงในเว็บไซต์ของเรา เว็บตรงสล็อตออนไลน์ของเรารองรับทุกแพลตฟอร์มและอุปกรณ์ทุกรุ่นทั้ง Android และ iOS ไม่ว่าคุณจะใช้โทรศัพท์ PC หรือแท็บเล็ตรุ่นไหน ก็อาจมั่นใจได้ว่าคุณจะหมุนสล็อตออนไลน์ได้อย่างไม่มีสะดุด ไม่มีความล่าช้าหรือสะดุดใด ๆ
ทดสอบเล่นสล็อตฟรี
เว็บตรงของเราเปิดให้บริการเพียงแค่คุณเข้ามาในเว็บไซต์ของ PG Slot ก็อาจทดลองเล่นสล็อตฟรีได้ทันที ไม่ต้องเสียค่าใช้จ่ายใด ๆ นี่เป็นโอกาสที่ดีในการฝึกฝนทักษะและเรียนรู้เกี่ยวกับเกมก่อนที่จะลงเดิมพันด้วยเงินจริง
การเล่นเกมสล็อตออนไลน์กับ PG Slot ช่วยให้คุณสามารถเพลิดเพลินกับการเล่นเกมคาสิโนได้ทุกที่ทุกเวลา ไม่ว่าคุณจะอยู่ที่ไหน เพียงแค่มีอุปกรณ์ที่เชื่อมต่ออินเทอร์เน็ต คุณก็จะสนุกกับการปั่นสล็อตได้อย่างไม่มีข้อจำกัด!
Год 2024 обещает стать особенным для поклонников азиатских сериалов. Новые дорамы 2024 года уже завоевывают сердца зрителей своими уникальными сюжетами и яркими персонажами. На сайте [url=https://doramaserials.net/doramy-2024/]doramaserials.net[/url] вы найдете самые ожидаемые новинки, которые подарят вам множество эмоций и незабываемых моментов. Удобный интерфейс сайта и регулярные обновления помогут вам всегда быть в курсе последних премьер и не пропустить ни одного интересного сериала.
На doramaserials.net доступны все [url=https://doramaserials.net/doramy-2024/]дорамы 2024 онлайн[/url], что позволяет наслаждаться любимыми шоу в любое удобное для вас время. Высокое качество видео и отличная озвучка делают просмотр еще более приятным и комфортным. Независимо от того, где вы находитесь, вы всегда сможете окунуться в мир захватывающих историй и погрузиться в атмосферу восточной культуры. Присоединяйтесь к миллионам поклонников и откройте для себя новые дорамы 2024 года!
the anticipation building as the plane climbs higher. The key is to cash out at the right moment before the aviator download app
На сайте https://filtry-dlya-ochistki-vody-gejzer.ru/ ознакомьтесь с техническими характеристиками фильтров для очистки воды Гейзер. Они сделают так, что в вашем доме всегда будет абсолютно чистая, полностью безопасная вода с безупречными органолептическими свойствами. Фильтры созданы в уникальных, лабораторных условиях, чтобы удовлетворить самые высокие предпочтения клиентов. Устройства обеспечивают безупречную степень очистки. При этом вода не будет слишком жесткой. Продукция реализуется по лучшей стоимости.
На сайте https://stroy-m2.ru/ вы сможете приобрести все для стройки, инструмент, профессиональное оборудование, а также все необходимое для обустройства дома, сада. Обязательно ознакомьтесь с предложениями дня, которые позволят вам существенно сэкономить свои финансы. Так у вас получится с хорошей скидкой приобрести все, о чем вы мечтали, включая садовую технику, а также все необходимое для ванной комнаты, организации благородной кухни. А если желаете найти что-то определенное, то воспользуйтесь специальным поиском.
https://pinup-oyun.com/ sayt?na daxil olun v? siz mind?n cox muxt?lif slot olan Pin Up onlayn platformas?na apar?lacaqs?n?z. Canl? oyunlar, klassik rulet, kazino, televiziya ?yl?nc?si v? daha cox. Sur?tli qeydiyyat, ayd?n interfeys v? ?la bonus sistemi
Go to the site https://miglandrealestate.com/ and you can find real estate on the island of Bali. Buying, renting land or villas and houses is very simple. We present the best offers at the best prices. We have the latest information about properties in Bali.
CVzen è il servizio di scrittura di CV https://cvzen.it/ e career coach per eccellenza per milioni di persone in cerca di lavoro in tutto il mondo. Il nostro team di esperti si impegna per il vostro successo e vi supporterà in tutto il vostro percorso professionale, dalla stesura di un CV professionale e di una lettera di presentazione specifica per il settore, all’ottimizzazione del vostro profilo LinkedIn, fino al career coaching professionale. Sbloccate nuove opportunità con un curriculum che metta in luce il vostro potenziale.
Over-betting: Betting too much too quickly can lead to significant losses. install aviator on pc
Купить дом Кипр. Все актуальные предложения о продаже домов, дач в Кипре в одном месте – всего размещено – 707 объявлений. Наиболее низкая стоимость продажи дома [url=https://overview-estate.ru/]стоимость дома на кипре[/url]
На сайте https://teletype.in/@registratoria77/kak-kupit-ili-prodat-gotovuu-firmu получите информацию о том, как реализовать или приобрести готовую компанию. Так вы узнаете, как приобрести нулевое ООО по привлекательной стоимости. Также есть информация и о том, как реализовать рабочую компанию с оборотами. Перед вами актуальная и ценная информация, которая ответит на все вопросы. Кроме того, вы будете в курсе того, как приобрести строительную компанию с историей, а также оборотами. Самые исчерпывающие рекомендации.
Found captivating reading that I’d like to recommend to everyone https://sportvlg.ru/user/Kapitonoviura
the social features, and the quality of the user interface. can we hack aviator game
Avtoshini.md предлагает купить шины по наилучшей цене. Мы постоянно обновляем ассортимент, новинки ждут вас. На товар предоставляем гарантию. У нас имеется то, что вам нужно. Для осуществления покупки, добавьте товар в корзину. Ищете шины 295/35 r20 в Молдове? Avtoshini.md – сайт, где имеется каталог, в нем представлена продукция известных производителей. Если появятся вопросы, свяжитесь с нашим грамотным менеджером для консультации. Звоните нам уже сейчас, мы с удовольствием поможем вам с приобретением и оформим заказ.
Интересуетесь смешанными единоборствами? На Octagon Express вы найдете [url=https://octagon.express/]главные новости ММА на сегодня[/url]. Мы публикуем самые свежие и важные новости, эксклюзивные интервью с бойцами, аналитические обзоры и результаты боев. Оставайтесь в курсе всех событий в мире ММА, следите за развитием карьеры ваших любимых бойцов и не пропускайте ни одной важной детали. Подписывайтесь на Octagon Express и всегда будьте в центре событий!
На сайте https://lodochnye-motory-77.ru/ представлены лодочные моторы в огромном выборе. Это позволит отыскать необходимую модель, которая подходит конкретно под ваши нужды. Тем самым вам будет обеспечен комфорт и удобство во время эксплуатации. Каждый товар прошел строгий контроль качества и соответствует самым высоким требованиям по безопасности. Предлагаются привлекательные, доступные расценки, а доставка заказа в самые разные регионы. Выбирая мотор, необходимо ориентироваться на мощность, а также тип топлива и то, какой вес у мотора.
На сайте https://split-sistemy-77.ru/ вы сможете приобрести качественные, функциональные, надежные сплит-системы, которые представлены здесь в огромном ассортименте. Они станут идеальным решением для домашнего и офисного использования. А если не можете определиться с выбором, то вам всегда помогут опытные и компетентные специалисты компании. Все устройства представлены проверенными, лучшими производителями. Они дают гарантии на все оборудование. Цель магазина заключается в том, чтобы ваша покупка была как можно приятней и экономически выгодной.
娛樂城
富遊娛樂城評價:2024年最新評價
推薦指數 : ★★★★★ ( 5.0/5 )
富遊娛樂城作為目前最受歡迎的博弈網站之一,在台灣擁有最高的註冊人數。
RG富遊以安全、公正、真實和順暢的品牌保證,贏得了廣大玩家的信賴。富遊線上賭場不僅提供了豐富多樣的遊戲種類,還有眾多吸引人的優惠活動。在出金速度方面,獲得無數網紅和網友的高度評價,確保玩家能享有無憂的博弈體驗。
推薦要點
新手首選: 富遊娛樂城,2024年評選首選,提供專為新手打造的豐富教學和獨家優惠。
一存雙收: 首存1000元,立獲1000元獎金,僅需1倍流水,新手友好。
免費體驗: 新玩家享免費體驗金,暢遊各式遊戲,開啟無限可能。
優惠多元: 活動豐富,流水要求低,適合各類型玩家。
玩家首選: 遊戲多樣,服務優質,是新手與老手的最佳賭場選擇。
富遊娛樂城詳情資訊
賭場名稱 : RG富遊
創立時間 : 2019年
賭場類型 : 現金版娛樂城
博弈執照 : 馬爾他牌照(MGA)認證、英屬維爾京群島(BVI)認證、菲律賓(PAGCOR)監督競猜牌照
遊戲類型 : 真人百家樂、運彩投注、電子老虎機、彩票遊戲、棋牌遊戲、捕魚機遊戲
存取速度 : 存款5秒 / 提款3-5分
軟體下載 : 支援APP,IOS、安卓(Android)
線上客服 : 需透過官方LINE
富遊娛樂城優缺點
優點
台灣註冊人數NO.1線上賭場
首儲1000贈1000只需一倍流水
擁有體驗金免費體驗賭場
網紅部落客推薦保證出金線上娛樂城
缺點
需透過客服申請體驗金
富遊娛樂城存取款方式
存款方式
提供四大超商(全家、7-11、萊爾富、ok超商)
虛擬貨幣ustd存款
銀行轉帳(各大銀行皆可)
取款方式
網站內申請提款及可匯款至綁定帳戶
現金1:1出金
富遊娛樂城平台系統
真人百家 — RG真人、DG真人、歐博真人、DB真人(原亞博/PM)、SA真人、OG真人、WM真人
體育投注 — SUPER體育、鑫寶體育、熊貓體育(原亞博/PM)
彩票遊戲 — 富遊彩票、WIN 539
電子遊戲 —RG電子、ZG電子、BNG電子、BWIN電子、RSG電子、GR電子(好路)
棋牌遊戲 —ZG棋牌、亞博棋牌、好路棋牌、博亞棋牌
電競遊戲 — 熊貓體育
捕魚遊戲 —ZG捕魚、RSG捕魚、好路GR捕魚、DB捕魚
Staying Calm: Keeping a cool head and avoiding impulsive decisions during gameplay. where can i play aviator
Leonbets – казино, заслуживающее внимания. Новичкам подойдет БК, потому как с ней просто разобраться. Много игр, быстрые выплаты, лояльный саппорт, выгодные акции и удобное мобильное приложение – все это радует. Ищете бк леон регистрация? Bettingsport.pro/brands/leon-bukmeker – сайт, где представлена только полезная информация, с ней ознакомиться можете в любое время. Чтобы делать ставки в букмекерской конторе Леон, необходимо зарегистрироваться. Удачи вам и крупных выигрышей!
Компания, специализирующаяся на продаже вилочных погрузчиков Heli https://heli.com.ru/ предлагает широкий ассортимент надежной и высокопроизводительной техники для погрузочно-разгрузочных работ. Heli – ведущий производитель вилочных погрузчиков, известный своим качеством и инновациями. Доверяйте профессионалам – выбирайте Heli для эффективной и безопасной работы на складе!
Mob25.com предоставляет для вас интересную и занимательную информацию. Здесь найдете новости, статьи и книги на различную тему. Узнаете, что можно засолить вместо селедки, как с помощью яркой плитки создать шедевры декора, почему француженки не носят Шанель, как сделать школьника отличником. Ищете изучение языков? Mob25.com – сайт, где у вас есть возможность найти каталог книг. У нас вы сможете избавиться от проблем с кожей и научитесь выбирать безопасную косметику. Имеется поиск, воспользуйтесь им прямо сейчас. Заходите на наш сайт с книгами в любое время!
На сайте https://uastend.com/messages/novyj-vzglyad-na-kontekstnuyu-reklamu-uastend-com-predlagaet-luchshie-marketingovye-resheniya/ вы сможете ознакомиться с увлекательной, интересной и полезной информацией, которая касается контекстной рекламы. Специально для вас самые эффективные, проверенные и надежные решения для продвижения бизнеса. При этом если вы вложите 100 долларов, то сможете заработать тысячу. Здесь рассматриваются и лучшие места для рекламы. А на первый месяц предоставляется скидка 50%.
На сайте https://kamdvor.ru/ вы сможете изучить весь ассортимент памятников, чтобы выбрать наиболее подходящий вариант. Есть возможность заказать такой памятник, который будет выполнен максимально быстро, особенно если нет времени ждать. В компании выполняют и фигурные гранитные надгробья, а также различные мемориальные комплексы. На все товары установлены доступные и привлекательны расценки, чтобы воспользоваться услугой смогли все желающие. Отсутствуют скрытые платежи. При необходимости специалист приедет абсолютно бесплатно.
Space Movers https://spacemovers.ca/ is renowned as the premier moving company in Calgary, Canada, offering unparalleled service and expertise in residential and commercial relocations. Their reputation as the best movers in the city is built on a foundation of reliability, efficiency, and a customer-centric approach that ensures a smooth and stress-free moving experience. Choosing Space Movers means choosing the best in the business, ensuring a seamless transition to your new home or office.
Are There Any Costs Involved? how to win aviator game
На сайте https://ofisnye-kresla-77.ru/ в большом выборе представлены офисные кресла самых разных расцветок, вариантов, а также модификаций. Все они созданы из качественных, современных и инновационных материалов, которые не утратят своего внешнего вида. Кресла идеально подходят как для установки дома, так и в офисе. С такими изделиями вы будете работать более плодотворно и функциональней. Все конструкции выполнены с учетом эргономических особенностей. Это особенно важно, если планируется долгая работа за столом.
Ищите прочистку канализации в Санкт-Петербурге? Зайдите на https://prochistka.ru/ где вы сможете воспользоваться услугами прочистки канализации, устранения засоров, заказать чистку водоснабжения и другие услуги. Огромный опыт, приятные цены, круглосуточный аварийный выезд. Вызвать сантехника устранить засор легко! Подробнее на сайте.
The basic rule of the Aviator Game is straightforward: place a bet, watch the plane take off, and decide when to aviator apps
Taker – это казино, которое пользуется невероятной популярностью. Сайт работает стабильно, без сбоев. Здесь представлен широкий выбор азартных игр. Если вы хотите отдохнуть и хорошо провести время, рекомендуем обратить внимание на Тейкер. Ищете такер? Vehrrxcso.ru – портал, предлагающий поиграть в удовольствие и при этом заработать хорошие деньги. Предоставляются щедрые акции и бонусы. Выплаты производятся без задержек, а служба поддержки быстро реагирует на обращения игроков. У Taker много поклонников, вы будете довольны работой казино.
Посмотреть любимый фильм или же дораму онлайн вместе с близкими и друзьями, даже находясь на расстоянии легко, у нас! Несколько движений – все, что необходимо для сотворения собственной кинокомнаты + делимся ссылкой на нее с другом! Ищете смотреть фильмы вместе с друзьями онлайн с чатом бесплатно без регистрации? Unotalone.su – сайт, где можно подробнее ознакомиться с информацией. Время таймлайна видео всех участников комнаты автоматически каждую секунду с ее владельцем синхронизируется. Помимо прочего, вы можете лично плеером управлять: регулировать качество и громкость, также ставить на паузу. Приятного вам просмотра!
сколько стоит стерилизовать кошку
где можно закодировать от алкоголизма https://lechenie-narkomanii.kz/
На сайте https://zazhigalki-77.ru/ в большом выборе находятся зажигалки, которые выполнены в традиционном, а также более современном направлении. Сотрудники магазина тщательно контролируют то, какая продукция поступает, чтобы клиенты смогли воспользоваться функциональными и высокотехнологичными решениями высокого качества. При заказе вы сможете ощутить все удобство покупки товаров через сайт, ведь здесь предусмотрен максимально комфортный интерфейс. Выбирайте бензиновые, газовые зажигалки, а также электрические.
It’s almost time! After catching this one at Fantastic Fest last year, we have been chomping at the bit for its wide release [url=https://www.dreadcentral.com/news/463211/possession-spreads-like-a-virus-in-new-when-evil-lurks-trailer-watch/]https://www.dreadcentral.com/news/463211/possession-spreads-like-a-virus-in-new-when-evil-lurks-trailer-watch/[/url]
В последние годы трейдинг на валютном рынке Форекс https://wilio.uz/ приобрел в Узбекистане огромную популярность. Этому способствует несколько факторов. Во-первых, доступность — для торговли нужен интернет, мобильный телефон и компьютер. То есть трейдинг в Ташкенте и Бухаре – это то же самое, что трейдинг в Самарканде и Намангане. Торговать можно из любой точки в любое время. Во-вторых, возможность заработка — трейдинг на Forex позволяет зарабатывать достаточно денег, чтобы сделать это своей профессией. В-третьих, низкий стартовый капитал — не требуется больших инвестиций, чтобы начать торговать.
Common Mistakes to Avoid aviator game news
tablets, making it convenient to play anywhere and anytime. aviator apk for pc
Заказать Вентилятор радиальный ВЦ 14-46 по оптовым ценам от завода производителя ООО Гевей можно на сайте https://vent-climate.ru/ Так же производим вентиляторы промышленные радиальные ВР 300-45.
Wonders Travel & Tourism: a https://jordan-travel.com agency located in Aqaba. Specializing in tours around Jordan, including Petra, Wadi Rum, the Dead Sea, and Amman. Offering private tours that can be customized to tourist interests and have positive reviews for professionalism and service.
Итальянская мебель от салона https://formul.ru в Москве – это большой выбор мебели из Италии по доступным ценам! Итальянская мебель в налиичи и на заказ. Купить итальянскую мебель в Москве по лучшим ценам.
Зайдите на https://freebet-master.info/ и вы сможете получить фрибет за регистрацию, фрибет без депозита, узнать букмекерские конторы с фрибетом, фрибет за регистрацию без депозита, бонусы бк, бонусы букмекерских контор, бонусы букмекеров, фрибет за регистрацию с депозитом, фрибет за регистрацию 2024, бесплатные фрибеты, фрибет за регистрацию, евро 2024, бонусы на евро 2024.
На сайте https://t.me/zaimys вы сможете взять в займы любую сумму. При этом деньги будут выданы в полном объеме на любые нужды. Обратиться сюда смогут даже те, у кого плохая кредитная история. Решение о выдаче средств принимается мгновенно. А потому нужная сумма будет выдана сразу же. Вы сможете потратить ее на свое усмотрение. При этом не нужно предоставлять справки, куда вы потратите деньги. Воспользуйтесь и вы возможностью получить нужную сумму прямо сейчас. Для этого просто оформите заявку, после чего сумма будет переведена на карточку.
FreeWorldTV.LIVE предоставляет большой выбор по всем миру телеканалов. И это совершенно бесплатно! Можно смотреть телеканалы на любом устройстве, подключенном к интернету, без необходимости регистрации или абонентской платы. https://freeworldtv.live/ – сайт, где имеется детальная информация, посмотрите ее уже сегодня. Вы познакомитесь с традициями, разными языками, стилями развлечений, и будете в курсе спортивных репортажей и мировых новостей. Получайте удовольствие от просмотра высококачественных HD-каналов!
На сайте https://t.me/zaimys вы сможете взять в займы любую сумму. При этом деньги будут выданы в полном объеме на любые нужды. Обратиться сюда смогут даже те, у кого плохая кредитная история. Решение о выдаче средств принимается мгновенно. А потому нужная сумма будет выдана сразу же. Вы сможете потратить ее на свое усмотрение. При этом не нужно предоставлять справки, куда вы потратите деньги. Воспользуйтесь и вы возможностью получить нужную сумму прямо сейчас. Для этого просто оформите заявку, после чего сумма будет переведена на карточку.
Multiplayer Options and Social Interaction how to win aviator game
Торговля на Форекс в Узбекистане https://instaforex.uz/ набирает обороты, открывая новые возможности для людей. Трейдинг в Ташкенте, Самарканде и в других городах помогает увеличить доход, защитить сбережения от инфляции и масштабировать финансовые возможности. Однако, будь то трейдинг в Намангане, Андижане или в другом городе, для прибыльной торговли на Forex нужен компьютер, интернет, знания и правильно выбранный брокер.
games that stood out from traditional casino offerings. Inspired by the concept of high-risk, high-reward, the aviator online game predictor tips and tricks
Found captivating reading that’s worth your time – take a look https://samtredia.com.ge/user/seaquester
Winline – отличная букмекерская контора. Понятный интерфейс, хорошие акции, быстрые выплаты – все это радует. Приложение работает без сбоев. Менеджеры службы технической поддержки стремятся реагировать оперативно для удовлетворения потребностей игроков. Вы можете разрешить проблемы с пополнением счета, выводом денежных средств. Ищете winline регистрация? Bettingsport.pro/brands/winline-bukmeker – здесь есть полезная информация, посмотрите ее уже сегодня. Предлагаем вам прекрасно провести свободное время. Играйте и наслаждайтесь!
Jetboos – достойная компания, которая обеспечивает для своих клиентов безопасность и организовывает частные перелеты. Предлагаем максимально выгодные цены и обязуемся соблюдать высокие стандарты защиты данных. Рады ответить на все ваши интересующие вопросы. Ищете заказ частного самолета? Jetboos.com – сайт, где можно прямо сейчас оставить заявку. Здесь также можете ознакомиться с отзывами о нас. Вы будете в восторге от сервиса и уровня комфорта. Забронируйте частный джет уже сейчас. Ваше путешествие будет беззаботным и приятным, доверьтесь нам!
Ищете актуальные прогнозы на сегодняшние бои UFC? Octagon Express представляет вам самые свежие [url=https://octagon.express/category/prognozy-na-ufc/]прогнозы UFC на сегодня[/url]! Наши аналитики тщательно изучают каждого бойца, чтобы предложить вам самые точные прогнозы. Следите за аналитикой и делайте ставки, опираясь на профессиональные мнения. Подписывайтесь на обновления и всегда будьте в курсе самых последних прогнозов на Octagon Express!
Origins of the Aviator Game aviator game hack download
LEMON CAR – автосервис с хорошей репутацией, который предоставляет услуги по приемлемой стоимости. Здесь трудится компетентная команда мастеров. Они используют только новейшее оборудование и готовы о вашей машине позаботиться. https://lemon-car.ru – сайт, где есть возможность узнать сроки и актуальные цены. Клиенты автосервиса на высшем уровне оценивают качество обслуживания и скорость. Позвоните нам уже сейчас и мы вас компетентно проконсультируем. Благодаря LemonCar ваш автомобиль будет ухоженно выглядеть, и работать надежно в течение длительного времени.
Olymp Trade https://olymptrade-app.online/ is an information resource designed to support and educate traders, offering access to a wide range of educational materials such as articles, video tutorials and webinars, as well as advanced analytical tools to help traders make informed decisions.
Подарок для конкурента
https://xrumer.ru/
Такой пакет берут для переспама анкор листа сайта или мощно усилить НЧ запросы
[url=https://xrumer.ru/]Подарок для конкурента[/url]
娛樂城評價
2024娛樂城推薦,經過玩家實測結果出爐Top5!
2024娛樂城排名是這五間上榜,玩家尋找娛樂城無非就是要找穩定出金娛樂城,遊戲體驗良好、速度流暢,Ace博評網都幫你整理好了,給予娛樂城新手最佳的指南,不再擔心被黑網娛樂城詐騙!
2024娛樂城簡述
在現代,2024娛樂城數量已經超越以前,面對琳瑯滿目的娛樂城品牌,身為新手的玩家肯定難以辨別哪間好、哪間壞。
好的平台提供穩定的速度與遊戲體驗,穩定的系統與資訊安全可以保障用戶的隱私與資料,不用擔心收到傳票與任何網路威脅,這些線上賭場也提供合理的優惠活動給予玩家。
壞的娛樂城除了會騙取你的金錢之外,也會打著不實的廣告、優惠滿滿,想領卻是一場空!甚至有些平台還沒辦法登入,入口網站也是架設用來騙取新手儲值進他們口袋,這些黑網娛樂城是玩家必須避開的風險!
評測2024娛樂城的標準
Ace這次從網路上找來五位使用過娛樂城資歷2年以上的老玩家,給予他們使用各大娛樂城平台,最終選出Top5,而評選標準為下列這些條件:
以玩家觀點出發,優先考量玩家利益
豐富的遊戲種類與卓越的遊戲體驗
平台的信譽及其安全性措施
客服團隊的回應速度與服務品質
簡便的儲值流程和多樣的存款方法
吸引人的優惠活動方案
前五名娛樂城表格
賭博網站排名 線上賭場 平台特色 玩家實測評價
No.1 富遊娛樂城 遊戲選擇豐富,老玩家優惠多 正面好評
No.2 bet365娛樂城 知名大廠牌,運彩盤口選擇多 介面流暢
No.3 亞博娛樂城 多語言支持,介面簡潔順暢 賽事豐富
No.4 PM娛樂城 撲克牌遊戲豐富,選擇多元 直播順暢
No.5 1xbet娛樂城 直播流暢,安全可靠 佳評如潮
線上娛樂城玩家遊戲體驗評價分享
網友A:娛樂城平台百百款,富遊娛樂城是我3年以來長期使用的娛樂城,別人有的系統他們都有,出金也沒有被卡過,比起那些玩娛樂城還會收到傳票的娛樂城,富遊真的很穩定,值得推薦。
網友B:bet365中文的介面簡約,還有超多體育賽事盤口可以選擇,此外賽事大部分也都有附上直播來源,不必擔心看不到賽事最新狀況,全螢幕還能夠下單,真的超方便!
網友C:富遊娛樂城除了第一次儲值有優惠之外,儲值到一定金額還有好禮五選一,實用又方便,有問題的時候也有客服隨時能夠解答。
網友D:從大陸來台灣工作,沒想到台灣也能玩到亞博體育,這是以前在大陸就有使用的平台,雖然不是簡體字,但使用介面完全沒問題,遊戲流暢、速度比以前使用還更快速。
網友E:看玖壹壹MV發現了PM娛樂城這個大品牌,PM的真人百家樂沒有輸給在澳門實地賭場,甚至根本不用出門,超級方便的啦!
Бесплатный хостинг картинок и обмен фотографиями для сайтов и блогов https://imgfoto.host/ это легкий способ и бесплатное решение для размещения картинок, скриншотов, фотографий по всему миру. Просто перетащите или вставьте изображение и готово! Можете воспользоваться и нашим API.
игровой автомат gates of olympus [url=https://gates-of-olympus-ru.ru/]gates-of-olympus-ru.ru[/url] .
На сайте https://xn—-utbcjbgv0e.com.ua/ вы найдете огромное количество компонентов, которые идеально подойдут для мыловарения, производства косметических средств. Здесь опубликовано все, что необходимо для того, чтобы творить и создавать полезную продукцию. Все товары от проверенных, надежных поставщиков, которые дорожат репутацией. При этом удерживаются привлекательные расценки, чтобы вы приобрели все необходимое. Возможны как оптовые, так и розничные продажи. Теперь и вы сможете создать свою качественную, натуральную косметику.
Player reviews and testimonials are overwhelmingly positive, with many praising the game’s exciting gameplay and aviator simulator casino
CVzen to najlepsza usługa pisania CV https://cvzen.pl/ i coach kariery dla milionów osób poszukujących pracy na całym świecie. Nasz zespół ekspertów jest zaangażowany w Twój sukces i będzie Cię wspierał przez całą Twoją karierę, od napisania profesjonalnego CV i listu motywacyjnego z uwzględnieniem specyfiki branży, po optymalizację profilu LinkedIn i zapewnienie profesjonalnego coachingu kariery. Odblokuj nowe możliwości dzięki CV, które pokaże Twój potencjał.
реристрация Dragon Money Casino сайт драгон мани казино
Портал о культуре Ярославля – ваш гид по культурной жизни города. Здесь вы найдёте информацию о театрах, музеях, галереях и исторических достопримечательностях. Откройте для себя яркие события, фестивали и выставки, которые делают Ярославль культурной жемчужиной России.
https://grand-sapphire-blu.com/
Grand Sapphire BLU Resort and Residences. Apartments for sale in Iskele,
Northern Cyprus. New launch by NorthernLand. Secure Your Apartment Today!
Several key developers and companies have played a significant role in the development and success of the demo do aviator
https://sunstyle.kiev.ua/
вход 1go casino бонус 1go casino
Overview of the Aviator Game Experience aviator app free download
Представьте себе платформу, где инновации и безопасность идут рука об руку. Этот проект – ваш проводник в будущее финансов. [url=http://kranmanipulator.com.ua/index.php?option=com_k2&view=itemlist&task=user&id=73684]http://kranmanipulator.com.ua/index.php?option=com_k2&view=itemlist&task=user&id=73684[/url]
Licensing and Regulation aviator gaming
На сайте https://kronshtejny-dlya-televizorov.ru/ вы сможете изучить кронштейны для телевизоров, которые представлены здесь в большом выборе. В разделе вы обязательно отыщите подходящее по форме, размеру решение. Есть фиксированные варианты, которые считаются наиболее надежными и практичными. А еще имеются наклонные, потолочные, поворотные. При выборе необходимо учесть размер телевизора, а также его вес. Все конструкции от лучших, проверенных марок, которые отвечают за качество.
Вас интересует профессиональная тонировка стекол автомобиля в Москве? Готовы помочь вам! Благодаря профессионализму специалистов и применению сертифицированных материалов, достигается высочайшее качество нашей деятельности. Вы можете смело нам доверять! http://xn—–6kccgdtutdbkoeocgd0azhek.xn--p1ai/ – сайт, где можете прямо сейчас оставить заявку. Мы обязательно свяжемся с вами в ближайшее время и ответим на ваши вопросы. Гарантируем привлекательные цены и часто проводим акции. Беремся за выполнение заказов любой сложности. Обращайтесь к нам в любое время!
https://lala.lanbook.com/virtualnaya-i-dopolnennaya-realnosti-v-obuchenii
дэдди казино официальный сайт
Привет студенты!
Всем привет) Будучи студентом, я наслаждался учебой до тех пор, пока не пришло время писать диплом. Но паниковать не стоило, ведь существуют компании, которые помогают с написанием и защитой диплома на отличные оценки!
Изначально я искал информацию по теме: купить красный диплом, купить аттестат старого образца, можно ли покупать диплом, купить дипломы о среднем образовании в москве, куплю диплом университета, затем наткнулся на https://www.arusak-diploms-srednee.ru/, где все мои учебные вопросы были решены!
Удачи и хороших оценок!
купить приложение к диплому
купить красный диплом
ли купить диплом вуза
можно ли купить аттестат в школе
где можно купить диплом о высшем образовании в рязани
дипломы награждения купить
купить диплом среднего профессионального образования
диплом о среднем профессиональном образовании купить в омске
купить диплом о высшем образовании с проведением
купить диплом о среднем профессиональном образовании в нижнем новгороде
На сайте https://xn--80aimfnww.xn--p1ai/ вы сможете воспользоваться такой популярной услугой, как кадровый аудит. В обязательном порядке будет оценен профессионализм, качество работы сотрудников. Кроме того, подготавливается к проверке ГИТ. Компетентные, лучшие специалисты найдут ошибки, которые были допущены при организации кадрового учета. Будут предложены максимально работающие методики их устранения. У сотрудников огромный опыт работы в данном направлении. Оставьте заявку на оказание услуги прямо сейчас!
крейзи манки [url=http://crazy-monkey-ru.ru/]крейзи манки[/url] .
User Interface and Design what is aviator game hack
квартира под цена купить квартиру в новостройке
Player台灣線上娛樂城遊戲指南與評測
台灣最佳線上娛樂城遊戲的終極指南!我們提供專業評測,分析熱門老虎機、百家樂、棋牌及其他賭博遊戲。從遊戲規則、策略到選擇最佳娛樂城,我們全方位覆蓋,協助您更安全的遊玩。
layer如何評測:公正與專業的評分標準
在【Player娛樂城遊戲評測網】我們致力於為玩家提供最公正、最專業的娛樂城評測。我們的評測過程涵蓋多個關鍵領域,旨在確保玩家獲得可靠且全面的信息。以下是我們評測娛樂城的主要步驟:
安全與公平性
安全永遠是我們評測的首要標準。我們審查每家娛樂城的執照資訊、監管機構以及使用的隨機數生成器,以確保其遊戲結果的公平性和隨機性。
02.
遊戲品質與多樣性
遊戲的品質和多樣性對於玩家體驗至關重要。我們評估遊戲的圖形、音效、用戶介面和創新性。同時,我們也考量娛樂城提供的遊戲種類,包括老虎機、桌遊、即時遊戲等。
03.
娛樂城優惠與促銷活動
我們仔細審視各種獎勵計劃和促銷活動,包括歡迎獎勵、免費旋轉和忠誠計劃。重要的是,我們也檢查這些優惠的賭注要求和條款條件,以確保它們公平且實用。
04.
客戶支持
優質的客戶支持是娛樂城質量的重要指標。我們評估支持團隊的可用性、響應速度和專業程度。一個好的娛樂城應該提供多種聯繫方式,包括即時聊天、電子郵件和電話支持。
05.
銀行與支付選項
我們檢查娛樂城提供的存款和提款選項,以及相關的處理時間和手續費。多樣化且安全的支付方式對於玩家來說非常重要。
06.
網站易用性、娛樂城APP體驗
一個直觀且易於導航的網站可以顯著提升玩家體驗。我們評估網站的設計、可訪問性和移動兼容性。
07.
玩家評價與反饋
我們考慮真實玩家的評價和反饋。這些資料幫助我們了解娛樂城在實際玩家中的表現。
娛樂城常見問題
娛樂城是什麼?
娛樂城是什麼?娛樂城是台灣對於線上賭場的特別稱呼,線上賭場分為幾種:現金版、信用版、手機娛樂城(娛樂城APP),一般來說,台灣人在稱娛樂城時,是指現金版線上賭場。
線上賭場在別的國家也有別的名稱,美國 – Casino, Gambling、中國 – 线上赌场,娱乐城、日本 – オンラインカジノ、越南 – Nhà cái。
娛樂城會被抓嗎?
在台灣,根據刑法第266條,不論是實體或線上賭博,參與賭博的行為可處最高5萬元罰金。而根據刑法第268條,為賭博提供場所並意圖營利的行為,可能面臨3年以下有期徒刑及最高9萬元罰金。一般賭客若被抓到,通常被視為輕微罪行,原則上不會被判處監禁。
信用版娛樂城是什麼?
信用版娛樂城是一種線上賭博平台,其中的賭博活動不是直接以現金進行交易,而是基於信用系統。在這種模式下,玩家在進行賭博時使用虛擬的信用點數或籌碼,這些點數或籌碼代表了一定的貨幣價值,但實際的金錢交易會在賭博活動結束後進行結算。
現金版娛樂城是什麼?
現金版娛樂城是一種線上博弈平台,其中玩家使用實際的金錢進行賭博活動。玩家需要先存入真實貨幣,這些資金轉化為平台上的遊戲籌碼或信用,用於參與各種賭場遊戲。當玩家贏得賭局時,他們可以將這些籌碼或信用兌換回現金。
娛樂城體驗金是什麼?
娛樂城體驗金是娛樂場所為新客戶提供的一種免費遊玩資金,允許玩家在不需要自己投入任何資金的情況下,可以進行各類遊戲的娛樂城試玩。這種體驗金的數額一般介於100元到1,000元之間,且對於如何使用這些體驗金以達到提款條件,各家娛樂城設有不同的規則。
квартиры в Санкт-Петербурге цены https://kvartiru-kupit-spb.ru
Наши металлические лестницы от производителя https://www.supersvarshik.com/ продуманы, просчитаны на прочность, с удобными ступенями из просечного листа, на которых не задерживается снег, песок или мусор. Ступени из металла и листа ПВЛ являются и прекрасным протектором, нога на них не скользит. Лестницы из металла и листа ПВЛ окрашены в камере порошковой краской – это защищенное и долговечное покрытие для металла. Установка и монтаж уличной металлической лестницы осуществляется на вкрученные в землю металлические сваи, на который методом сварки крепятся блоки из площадки и лестничного марша, Монтаж уличной лестницы из металла проходит очень оперативно, в отличии от других видов лестниц. Спроектируем, изготовим и смонтируем для вас самую правильную лестницу!
台灣線上娛樂城
在现代,在线赌场提供了多种便捷的存款和取款方式。对于较大金额的存款,您可以选择中国信托、台中银行、合作金库、台新银行、国泰银行或中华邮政。这些银行提供的服务覆盖金额范围从$1000到$10万,确保您的资金可以安全高效地转入赌场账户。
如果您需要进行较小金额的存款,可以选择通过便利店充值。7-11、全家、莱尔富和OK超商都提供这种服务,适用于金额范围在$1000到$2万之间的充值。通过这些便利店,您可以轻松快捷地完成资金转账,无需担心银行的营业时间或复杂的操作流程。
在进行娱乐场提款时,您可以选择通过各大银行转账或ATM转账。这些方法不仅安全可靠,而且非常方便,适合各种提款需求。最低提款金额为$1000,而上限则没有限制,确保您可以灵活地管理您的资金。
在选择在线赌场时,玩家评价和推荐也是非常重要的。许多IG网红和部落客,如丽莎、穎柔、猫少女日记-Kitty等,都对一些知名的娱乐场给予了高度评价。这些推荐不仅帮助您找到可靠的娱乐场所,还能确保您在游戏中享受到最佳的用户体验。
总体来说,在线赌场通过提供多样化的存款和取款方式,以及得到广泛认可的服务质量,正在不断吸引更多的玩家。无论您选择通过银行还是便利店进行充值,都能体验到快速便捷的操作。同时,通过查看玩家的真实评价和推荐,您也能更有信心地选择合适的娱乐场,享受安全、公正的游戏环境。
На сайте http://nemans.ru вы сможете приобрести всю необходимую продукцию, включая пакеты с ручками, фасовочные пакеты, мешки полипропиленовые, пленку армированную, полиэтиленовую. Также в разделе имеется ветошь, ароматное мыло, перчатки, черенки, бытовая химия, ведра и многое другое, что вы сможете заказать прямо сейчас. Среди рекомендованных товаров находятся вязаные перчатки, туалетная бумага, хозяйственное мыло, неткол. Вся продукция отличного качества, надежная и наделена длительными сроками эксплуатации.
Social and Community Aspects aviator app free download
Opened up interesting material Р I recommend sharing this discovery http://lolipopnews.ru/poluchite-besplatnyie-frispinyi-na-luchshie-slotyi
Found an enthralling article, I recommend you to read https://bbbbnewss.blogspot.com/2024/06/blog-post_88.html
На сайте https://top-volna.ru воспользуйтесь уникальной возможностью послушать любимую радиостанцию и просто получить отменное настроение от приятно проведенного времени. На этом портале такие радиостанции, как: Маруся ФМ, Русское Радио, Новое Радио, Европа Плюс, DFM, Радио Энерджи, Ретро FM, Юмор FM и многое другое. Прямо сейчас послушайте самые популярные песни, которые нравятся многим. Вас покорит отличное звучание, а также интересная аранжировка, которая точно никого не оставит без внимания.
На сайте https://elektrovelosipedy-77.ru/ представлен внушительный выбор электровелосипедов различных марок. Они идеально подойдут тем, кто любит активно отдыхать. Это очень комфортный, удобный способ передвижения, который поможет вам сэкономить много времени. На всю продукцию действуют привлекательные цены, а доставка происходит максимально оперативно. Окончательно определиться с покупкой помогут технические характеристики, которые и влияют на цели покупки. При покупке необходимо полагаться и на емкость батареи.
На сайте https://www.parvenik.ru/ представлены веники в большом ассортименте и из самого разного дерева. Есть как дубовые, так и березовые, хвойные, эвкалиптовые. Имеются варианты из липы, клена, травы в пучках и многое другое. Представлены эфирные масла, чаи для бани и многое другое, что необходимо для того, чтобы оздоровить организм, полноценно попариться в бане. Очень часто на сайте проходят акции, действуют скидки. Приобретайте товары со скидками. Перед покупкой обязательно ознакомьтесь с характеристиками.
Variety: Access to a wide range of betting options and gameplay modes. aviator predictor demo
Скважина под питьевую воду Иркутск мастер-буров.рф
Компания Мастер Буров представляет сервис по бурению скважин на Вашем загородном участке в Иркутске и Иркутской области. Работая в представленной области уже множество лет, мы знаем все тонкости и даем гарантию на выполненную работу в срок 10 лет. А вдобавок, у нас есть рассрочка без процентов на три месяца, что очень удобно, если нет свободных денежных средств на настоящий момент. На сайте мастер-буров.рф ознакомьтесь с нашими расценками, примерами скважин и иной важной информацией.
По вопросу [url=https://xn—-7sbeem4bsncflq.xn--p1ai/]скважина иркутск цена[/url] мы вам непременно окажем помощь. На представленном веб ресурсе можно узнать примерную глубину бурения на своем участке в видео, а также стоимость. Но самый правильный метод — это позвать к себе эксперта для выявления лучшего места под скважину, понятие объема работы и, в соответствии с этим, прайс. Оставьте свой номер телефона и мы свяжемся с Вами в ближайшее время.
У нас личная техника с буровыми установками, которая предоставляет возможность оказывать услуги высокого качества по очень выгодным расценкам. В распоряжении 8 единиц техники и 22 профессионала с положительным опытом. Вы также можете купить всё самое нужное оборудование для водоснабжения. Среди них: химический анализ воды, набор для подключения, насос для скважины, септик и другое. По оставшимся вопросам звоните по контактному телефону 8(3952)559-589 или приезжайте по адресу: г. Иркутск, мкр-н Радужный, д. 12, оф. 310.
Приобрести [url=https://xn—-7sbeem4bsncflq.xn--p1ai/]скважина под питьевую воду[/url] можно на веб портале мастер-буров.рф прямо сейчас. Скважина с питьевой водой — один из главных элементов на Вашем участке. Мы даем гарантию на качество работы, материалов, а также предлагаем сервисное обслуживание. Природные факторы могут плохо влиять на скважину и качество воды, мы также справляемся с восстановлением скважин от деформации и с очищением от заиливания. Звоните, приходите, будем рады с Вами работать.
На сайте https://napolnye-plintusy.ru/ в огромном ассортименте представлены напольные плинтусы. Они станут идеальным решением для декорирования помещения. В каталоге вы найдете: деревянные, пластиковые, металлические плинтусы, а также те, что выполнены из МДФ. Перед вами огромный ассортимент цветовой палитры, а также модификаций. Есть как светлые, так и темные, яркие решения, которые станут яркой деталью стиля. Важным моментом является то, что изделия неприхотливы в уходе и не утратят технических характеристик длительное время.
Посетите сайт https://seovgeo.ru/ где вы сможете заказать продвижение на яндекс картах, продвижение компании в яндекс картах, продвижение организации на яндекс картах, сео продвижение на яндекс картах, продвижение через яндекс карты и многие другие услуги с индивидуальным подходом от профессионалов по выгодным ценам. Подробнее на сайте.
купить квартиру в Санкт-Петербурге https://kvartiru-kupit78.ru
https://kupit-kvartiruspb.ru в новостройках Санкт-Петербурга. Цены и фотографии квартир от застройщика в готовых и строящихся ЖК. Подбор жилья, ипотечные программы, сопровождение сделок и выгодные предложения.
http://prikoly-tut.ru/
Player台灣線上娛樂城遊戲指南與評測
台灣最佳線上娛樂城遊戲的終極指南!我們提供專業評測,分析熱門老虎機、百家樂、棋牌及其他賭博遊戲。從遊戲規則、策略到選擇最佳娛樂城,我們全方位覆蓋,協助您更安全的遊玩。
layer如何評測:公正與專業的評分標準
在【Player娛樂城遊戲評測網】我們致力於為玩家提供最公正、最專業的娛樂城評測。我們的評測過程涵蓋多個關鍵領域,旨在確保玩家獲得可靠且全面的信息。以下是我們評測娛樂城的主要步驟:
安全與公平性
安全永遠是我們評測的首要標準。我們審查每家娛樂城的執照資訊、監管機構以及使用的隨機數生成器,以確保其遊戲結果的公平性和隨機性。
02.
遊戲品質與多樣性
遊戲的品質和多樣性對於玩家體驗至關重要。我們評估遊戲的圖形、音效、用戶介面和創新性。同時,我們也考量娛樂城提供的遊戲種類,包括老虎機、桌遊、即時遊戲等。
03.
娛樂城優惠與促銷活動
我們仔細審視各種獎勵計劃和促銷活動,包括歡迎獎勵、免費旋轉和忠誠計劃。重要的是,我們也檢查這些優惠的賭注要求和條款條件,以確保它們公平且實用。
04.
客戶支持
優質的客戶支持是娛樂城質量的重要指標。我們評估支持團隊的可用性、響應速度和專業程度。一個好的娛樂城應該提供多種聯繫方式,包括即時聊天、電子郵件和電話支持。
05.
銀行與支付選項
我們檢查娛樂城提供的存款和提款選項,以及相關的處理時間和手續費。多樣化且安全的支付方式對於玩家來說非常重要。
06.
網站易用性、娛樂城APP體驗
一個直觀且易於導航的網站可以顯著提升玩家體驗。我們評估網站的設計、可訪問性和移動兼容性。
07.
玩家評價與反饋
我們考慮真實玩家的評價和反饋。這些資料幫助我們了解娛樂城在實際玩家中的表現。
娛樂城常見問題
娛樂城是什麼?
娛樂城是什麼?娛樂城是台灣對於線上賭場的特別稱呼,線上賭場分為幾種:現金版、信用版、手機娛樂城(娛樂城APP),一般來說,台灣人在稱娛樂城時,是指現金版線上賭場。
線上賭場在別的國家也有別的名稱,美國 – Casino, Gambling、中國 – 线上赌场,娱乐城、日本 – オンラインカジノ、越南 – Nhà cái。
娛樂城會被抓嗎?
在台灣,根據刑法第266條,不論是實體或線上賭博,參與賭博的行為可處最高5萬元罰金。而根據刑法第268條,為賭博提供場所並意圖營利的行為,可能面臨3年以下有期徒刑及最高9萬元罰金。一般賭客若被抓到,通常被視為輕微罪行,原則上不會被判處監禁。
信用版娛樂城是什麼?
信用版娛樂城是一種線上賭博平台,其中的賭博活動不是直接以現金進行交易,而是基於信用系統。在這種模式下,玩家在進行賭博時使用虛擬的信用點數或籌碼,這些點數或籌碼代表了一定的貨幣價值,但實際的金錢交易會在賭博活動結束後進行結算。
現金版娛樂城是什麼?
現金版娛樂城是一種線上博弈平台,其中玩家使用實際的金錢進行賭博活動。玩家需要先存入真實貨幣,這些資金轉化為平台上的遊戲籌碼或信用,用於參與各種賭場遊戲。當玩家贏得賭局時,他們可以將這些籌碼或信用兌換回現金。
娛樂城體驗金是什麼?
娛樂城體驗金是娛樂場所為新客戶提供的一種免費遊玩資金,允許玩家在不需要自己投入任何資金的情況下,可以進行各類遊戲的娛樂城試玩。這種體驗金的數額一般介於100元到1,000元之間,且對於如何使用這些體驗金以達到提款條件,各家娛樂城設有不同的規則。
На сайте http://koleso-na-hodu.ru каждый желающий получает возможность приобрести шины больших, а также редких размеров с оперативной доставкой по всему городу, области. Непосредственно на сайте у вас получится подобрать шины, исходя из таких параметров, как: ширина, высота профиля, диаметр, стоимость, производитель, сезонность. Вся продукция сертифицированная, качественная, оригинальная. Если остались вопросы, то заполните специальную форму со своими данными, чтобы менеджер перезвонил для уточнения определенных моментов.
娛樂城
在现代,在线赌场提供了多种便捷的存款和取款方式。对于较大金额的存款,您可以选择中国信托、台中银行、合作金库、台新银行、国泰银行或中华邮政。这些银行提供的服务覆盖金额范围从$1000到$10万,确保您的资金可以安全高效地转入赌场账户。
如果您需要进行较小金额的存款,可以选择通过便利店充值。7-11、全家、莱尔富和OK超商都提供这种服务,适用于金额范围在$1000到$2万之间的充值。通过这些便利店,您可以轻松快捷地完成资金转账,无需担心银行的营业时间或复杂的操作流程。
在进行娱乐场提款时,您可以选择通过各大银行转账或ATM转账。这些方法不仅安全可靠,而且非常方便,适合各种提款需求。最低提款金额为$1000,而上限则没有限制,确保您可以灵活地管理您的资金。
在选择在线赌场时,玩家评价和推荐也是非常重要的。许多IG网红和部落客,如丽莎、穎柔、猫少女日记-Kitty等,都对一些知名的娱乐场给予了高度评价。这些推荐不仅帮助您找到可靠的娱乐场所,还能确保您在游戏中享受到最佳的用户体验。
总体来说,在线赌场通过提供多样化的存款和取款方式,以及得到广泛认可的服务质量,正在不断吸引更多的玩家。无论您选择通过银行还是便利店进行充值,都能体验到快速便捷的操作。同时,通过查看玩家的真实评价和推荐,您也能更有信心地选择合适的娱乐场,享受安全、公正的游戏环境。
Chasing Losses: Trying to recover losses by betting more can be detrimental. aviator game online india
Зайдите на сайт https://365-calendar.ru/ и вы сможете узнать все о винограде – виноградарство, сорта винограда, выращивание и уход, а советы и рекомендации опытных виноградарей помогут вам достичь успеха в выращивании виноградных лоз. Вы сможете не только прочитать информацию о сортах винограда и выращивании, но также найти новости из мира виноделия с интересными фактами. Подробнее не сайте.
керамические блоки
The Aviator Game boasts several unique features that set it apart from other online casino games: aviator game download for pc
На сайте https://climat.best приобретите качественные, функциональные кондиционеры, сплит-системы, тепловые насосы, а также чиллеры. Вся продукция именитых, проверенных и надежных брендов, а потому техника прослужит долго без потери технических характеристик, возможностей. А если необходимо подобрать что-то определенное, то воспользуйтесь специальным поиском. Перед вами есть и самые популярные позиции, которые пользуются особенным спросом среди покупателей. В интернет-магазине вы отыщите как настенные, так и напольно-потолочные конструкции, кассетные.
квартиры в Санкт-Петербурге цены https://novostroyki-spb78.ru
Summary of Key Points how to beat the aviator game
На сайте https://uss.eu.com изучите интересную, увлекательную и свежую информацию на самую разную тему. Здесь опубликован материал о красивых украшениях. Предлагается ознакомиться с бестселлерами. Также есть данные о том, как правильно увеличить свой охват в Интернете. Имеется полный список компаний, которые пригодятся владельцам предприятий. Обязательно ознакомьтесь со списком лучших форумов, которые представлены в Интернете. Опубликованы и самые лучшие, проверенные англоязычные форумы, на которые вы сможете зайти прямо сейчас.
Variety: Access to a wide range of betting options and gameplay modes. aviator game tricks
На сайте https://pd-seller.ru получится скачать бесплатно либо приобрести Parallels Desktop 19 для Mac. Предоставляется месячная гарантия возврата денежных средств. Вас ожидает огромное количество различных приложений, которые используются для Windows, торговое, бухгалтерское ПО, КОМПАС и многое другое. Посмотрите видео-презентацию прямо сейчас! При покупке вы получите ключ Windows абсолютно бесплатно. Все лицензии являются официальными. Вы сможете легко приобрести, воспользоваться, продлить и обновить.
Доставка цветов и подарков по городу Минску https://sendflowers.by/ Беларуси и миру! Мы предлагаем посетителям доставку роз, букетов цветов, цветочные композиции, дополненные изысканными конфетами, воздушными шарами, яркими открытками, вазами, а также мишками, мягкими игрушками, открытками ручной работы в Минске и во всех городах Беларуси. Международная доставка цветов через партнерскую сеть Telelfora.
Каталог эротических рассказов https://vicmin.ru подарит тебе возможность уйти от рутины и погрузиться в мир секса и безудержного наслаждения. Обширная коллекция рассказов для взрослых разбудит твое воображение и принесет немыслимое удовольствие.
Discovered a unique article – recommended to acquaint yourself! https://israelafrica.mn.co/spaces/9350420/feed
Ищете самые свежие и сенсационные новости из мира смешанных единоборств? Octagon Express предлагает вам [url=https://octagon.express/category/novosti-mma/]новости ММА[/url]! Узнайте первыми о последних боях, интервью с бойцами, аналитических обзорах и эксклюзивных видео. Будьте в курсе всех значимых событий и не пропустите ни одной сенсации. Подписывайтесь на обновления и следите за всеми новостями ММА вместе с Octagon Express!
Unique Features of the Aviator Game aviator game 1xbet hack
G-Raze компания изготавливает надежные и мощные вездеходы, которые можно купить по лояльной стоимости. Мы те производители, которые после приобретения предлагают консультацию на бесплатной основе. С нами вы в каждой поездке ощутите уверенность и комфорт. Ищете вездеход болотоход цены? G-raze.ru – сайт, где можно увидеть модельный ряд вездеходов и видеообзор. Доступные цены, отменное качество, комфорт – вот наши основные достоинства. Если вам интересен наш проект, оставьте заявку, и мы вам перезвоним. У нас лучшее качество вездеходов. Удостоверьтесь в этом сами.
На сайте https://esdogames.ru ознакомьтесь с самыми трендовыми, актуальными и интересными играми, которые точно произведут на вас эффект. Здесь вы найдете все, что вас интересует, включая самые зрелищные игры, которые захватывают с самых первых минут. Также представлен список лучших игр за 2024 год. Вы сможете поиграть во все, что хочется, прямо сейчас. А если вы ищите что-то конкретное, то воспользуйтесь специальным поиском. Подберите игру по жанру, режиму игры, платформе, чтобы облегчить поиск.
На сайте https://usiic.co почитайте интересные и увлекательные материалы, посвященные высоким технологиям. Все они написаны экспертами, которые отлично разбираются в таких темах. Есть данные на тему лучших игральных ноутбуков, которые отличаются особой мощностью, производительностью и стильным дизайном. Опубликован список самых эффективных и прогрессивных из них. Есть информация про вечный двигатель, оснащенный магнитами. Вы узнаете про то, как выполнить быстрый и эффективный ремонт мягкой кровли.
the thrill of betting and cashing out. Common themes in reviews include the game’s fairness, the enjoyment of aviator game link
На сайте http://waltzprof.com закажите обратный звонок с той целью, чтобы ознакомиться с полным ассортиментом, который производит завод ООО “Валцпроф”. Здесь представлен стальной профиль в большом выборе. Изучите технические характеристики фасадной системы, которая создана из нержавеющей, оцинкованной стали. Также в компании получится приобрести и скрытые петли для профильных дверей. Вся продукция создается на высокотехнологичном, инновационном оборудовании. Выпускается только продукция высокого качества и в строго обозначенные сроки.
משתמשים בשירותיהן. אז מי בעצם צריך נערות ליווי? מי צריך נערות? אנשי עסקים – ירושלים היא מרכז עסקים חשוב מאוד ואנשי עסקים מהארץ ולכל אחת יש את הקסם שלה. יש כאן סטודנטיות ישראליות שובבות, לצד אירופאיות עדינות או לטיניות לוהטות. אתה בוחר את הבחורה, ולאחר מכוני ליווי בחיפה – למה לבחור דווקא בבחורות החדשות בחבורה?
Новые технологии и материалы – достойная компания, которая специализируется на изготовлении для радиотехнической промышленности комплектующих. Предлагаем вам по заманчивой стоимости приобрести метизы и крепеж. Внимательно следим за своей продукцией и поставляем ее точно в срок. Готовы предоставить вам помощь в оформлении заказа. Ищете заклепки гост купить? Ntmsp.ru – сайт, где вы можете уже сегодня ознакомиться с акциями. У нас работают только опытные и квалифицированные специалисты. С удовольствием ответим на все ваши вопросы, свяжитесь с нами.
Исламский информационный портал https://naistine.ru/ рассказывающий о религии Ислам. На сайте вы найдете ценные советы, различные молитвы, аяты и суры из Корана, а так же достоверные хадисы.
На сайте https://setevoj-kabel.ru/ в большом многообразии находятся сетевые кабели. Они отлично подходят для самых разных целей. Они обеспечат качественное, надежное подключение, а потому необходимы везде. В магазине вы отыщите варианты самых разных типов и только от проверенных, лучших брендов. При выборе необходимо учитывать то, для каких целей будет использоваться кабель и в каких условиях. Также на покупку влияют и такие моменты: длина кабеля, уровень экранирования и другие нюансы. Вы всегда сможете воспользоваться консультацией специалиста.
https://pet.fish/community/profile/gayemcmichael4/
https://toripedia.info/index.php/User:FrancescoHorgan
Effective Strategies for Beginners pin up casino aviator
Агентство по продвижению телеграм-каналов https://883666b.com в Москве специализируется на разработке и реализации стратегий для увеличения аудитории и вовлечённости подписчиков на телеграм-каналах. Эксперты агентства помогают клиентам определить целевую аудиторию, разрабатывают контент-планы и рекламные кампании. Услуги включают рекламу посевами, таргет рекламой, анализ конкурентов, SEO-оптимизацию контента.
Scoring System and Winning Criteria strategy for aviator game
בודי בשמנים חמים וארומטיים או טיפול פרוסטטה לגברים. כמו כן בשנים האחרונות הרבה זוגות נשואים באים לקליניקות פרטיות על מנת להתפנק אך לבתי המלון ישנם גם חסרונות! מהמונח דיסקרטיות אתם יכולים לשכוח, אין שם דיסקרטיות בבת המלון; שם רואים בדיוק מי מגיע 24 שעות דירה דיסקרטית במרכז
купить аттестат за 9 класс [url=http://www.school5-priozersk.ru]http://www.school5-priozersk.ru[/url] .
[url=http://www.peregonavtofgtd.kiev.ua]www.peregonavtofgtd.kiev.ua[/url]
Я мухой, сверхэффективно и еще надежно перебросить Ваш автомобиль с Украины в Европу, чи с Европы в течение Украину хором начиная с. ant. до нашей командой. Формирование доказательств и вывоз производятся в течение оговоренные сроки.
https://peregonavtofgtd.kiev.ua
Москва Купить Мефедрон? Кристаллы МЕФ?
Где в Москве Купить Мефедрон? САЙТ – https://mephedrone.top/
את חיי האינטימיות שלכם. פצצות מוכשרות שיעשו הכל עבור החבר על מנת לספק לו עיסוי אירוטי בצפון ורגיעה מוחלטת, נפגשות עם גברים וגם מציעות. אם לבחור יש נכס משלו, הנשים יבואו לשם מרצון, על מנת לספק הנאה מדהימה ולמלא את כל משאלותיו. עם זאת, הבנות גם יכולות Adorable girls from escorts services
History and Development aviator hack apk download pc
Результат работы студии «Мята» – идеальный интерьер, который полностью соответствует вашим ожиданиям. Наша команда состоит из профессиональных специалистов. Предлагаем наилучшие цены на дизайн, гарантируем компетентность и ответственность. Мы завоевали доверие наших клиентов. Ищете дизайн проект квартиры в ташкенте? Tashkent.studio-mint.pro – сайт, где представлены отзывы о студии, прочитайте их прямо сейчас. Нас рекомендуют знакомым и друзьям. Чтобы заказать дизайн интерьера, позвоните нам. Открыты к диалогу на протяжении всего проекта. Исполним вашу мечту!
LEMON CAR – автосервис с хорошей репутацией, который предоставляет услуги по приемлемой стоимости. Здесь трудится компетентная команда мастеров. Они применяют исключительно современное оборудование и всегда готовы позаботиться о вашей машине. https://lemon-car.ru – сайт, где есть возможность узнать сроки и актуальные цены. Клиенты автосервиса на высшем уровне оценивают качество обслуживания и скорость. Позвоните нам, и мы дадим вам грамотную консультацию. Благодаря LemonCar ваш автомобиль будет ухоженно выглядеть, и работать надежно в течение длительного времени.
Discovered a unique article – recommended to acquaint yourself! http://elektrofahrrad-tests.de/forums/newthread.php?fid=2&processed=1
casino game. aviator slot
Зайдите на сайт https://generalsfaq.ru/ который создан для поклонников компьютерной стратегии Генералы. Вы сможете почитать FAQ и гайды по игре, найти карты Zero Hour, а также прочие военные стратегии. Generals это лучшая RTS стратегия для компьютера – присоединяйтесь к нашему сообществу!
На сайте https://shvejnye-mashiny-77.ru/ ознакомьтесь со всем ассортиментом швейных машинок, которые различаются не только внешним видом, дизайном, но и техническими параметрами, стоимостью. А для того, чтобы сделать правильный выбор, следует ознакомиться с характеристиками каждого изделия. Для всех клиентов действует оперативная доставка, установлены привлекательные цены, предоставляются гарантии. При выборе следует уточнить навыки шитья, уточнить то, какие опции вам нужны. Действуют разумные цены.
На сайте https://kupit-roboty-pylesosy.ru/ в большом выборе представлены роботы-пылесосы, которые порадуют своей качественной работой, функциональностью. Многие из них идеально подходят как для сухой, так и влажной уборки. На устройства предоставляются гарантии, они отличаются длительным сроком эксплуатации. При выборе необходимо учесть не только тип помещения, но и то, есть ли у вас домашние животные, то, какого размера помещение, то, сколько будет работать устройство. Выбирайте изделие, полагаясь на эти простые рекомендации.
7k casino скачать приложение
The Popularity of Online Casino Games aviator tool hack download
Came across an interesting article, worth a glance https://www.scoop.it/topic/lara-by-lara-87/p/4153704363/2024/06/14/hamster-kombat
MEBELZOOM предлагает только высококачественную продукцию. Все изделия сертифицированы и соответствуют всем необходимым стандартам РФ. Вы можете связаться с нашей службой поддержки при возникновении вопросов. У нас трудятся специалисты с приличным опытом работы в мебельном бизнесе. Ищете купить стол на кухню недорого? Mebelzoom.ru – сайт, где есть возможность найти широкий выбор мебели. Гарантируем привлекательные цены, высокое качество товаров и оперативную доставку. Обращайтесь к нам, мы готовы предоставить вам квалифицированную помощь.
ремонт кофемашин bosch
Cristiano Ronaldo https://cristiano-ronaldo.prostoprosport-ar.com is a Portuguese footballer, forward, captain of the Saudi Arabian club An-Nasr and the Portuguese national team. European Champion. Considered one of the best football players of all time. The best scorer in the history of football according to the IFFIS and fourth according to the RSSSF
Официальный городской информационный портал – новости Ижевска и Удмуртии, криминал, дорожно-транспортные происшествия (ДТП), чрезвычайные происшествия(ЧП) [url=https://vk.com/mdps18]https://vk.com/mdps18[/url]
недорогое продвижение сайтов в москве [url=https://prodvizhenie-sajtov-v-moskve117.ru/]prodvizhenie-sajtov-v-moskve117.ru[/url] .
Anderson Sousa Conceicao better known as Talisca https://talisca.prostoprosport-ar.com is a Brazilian footballer who plays as a midfielder for the An-Nasr club. A graduate of the youth team from Bahia, where he arrived in 2009 ten years ago.
Yassine Bounou https://yassine-bounou.prostoprosport-ar.com also known as Bono, is a Moroccan footballer who plays as a goalkeeper for the Saudi Arabian club Al-Hilal and the Moroccan national team. On November 10, 2022, he was included in the official application of the Moroccan national team to participate in the matches of the 2022 World Cup in Qatar
vavada зеркало на сегодня
На сайте https://pharmspirt.ru/ вы сможете подобрать уникальные и революционные решения из сферы хранения спиртов, прекурсоров, ЛВЖ. Среди основных сфер деятельности выделяют: оказание услуг для фармацевтических, химических, пищевых компаний. Предприятие оказывает профессиональный консалтинг, связанный с правилами хранения этилового спирта. Компания проводит и обучение: вебинары в сфере оборота спирта, спиртосодержащей продукции. Сотрудники прекрасно понимают программу, потому как она подается в простой форме.
Encountered a captivating article, I propose you read https://planetasp.ru/forum/viewtopic.php?pid=17125#p17125
Pingback: 5-day prednisone dosage for bronchitis
ремонт телефонов в москве
Harry Edward Kane https://harry-kane.prostoprosport-ar.com is an English footballer, forward for the German club Bayern and captain of the England national team. Considered one of the best football players in the world. He is Tottenham Hotspur’s and England’s all-time leading goalscorer, as well as the second most goalscorer in the Premier League. Member of the Order of the British Empire.
Искал автошколу в своем районе у м. Аэропорт или Сокол, там одна улица проходит. Считаю, что сделал неплохой выбор [url=http://gai-18.ru]автошкола в москве недорого[/url]
Neymar da Silva Santos Junior https://neymar.prostoprosport-ar.com is a Brazilian footballer who plays as a striker, winger and attacking midfielder for the Saudi Arabian club Al-Hilal and the Brazilian national team. Considered one of the best players in the world. The best scorer in the history of the Brazilian national team.
Достижение положительного результата в освоении управления легковым автомобилем и получении водительских прав гарантирует автошкола с репутацией [url=http://avtopoisk43.ru/]автошкола белорусская[/url]
Erling Breut Haaland https://erling-haaland.prostoprosport-ar.com is a Norwegian footballer who plays as a forward for the English club Manchester City and the Norwegian national team. English Premier League record holder for goals per season.
ремонт стиральных машин гагенау официальный сервисный
Pingback: use of ampicillin and cloxacillin
Pingback: do trazodone side effects go away
Ali al-Buleahi https://ali-al-bulaihi.prostoprosport-ar.com Saudi footballer, defender of the club ” Al-Hilal” and the Saudi Arabian national team. On May 15, 2018, Ali al-Buleakhi made his debut for the Saudi Arabian national team in a friendly game against the Greek team, coming on as a substitute midway through the second half.
Самый читаемый секс блог о жизни: https://sexcloud.ru/
квартирные переезды с грузчиками дешево [url=pereezdminsk.ru]квартирные переезды с грузчиками дешево [/url] .
Luka Modric https://lukamodric.prostoprosport-ar.com is a Croatian footballer, central midfielder and captain of the Spanish club Real Madrid, captain of the Croatian national team. Recognized as one of the best midfielders of our time. Knight of the Order of Prince Branimir. Record holder of the Croatian national team for the number of matches played.
Зайдите на сайт Завода РемонтЯм https://xn--e1aoadejs8h.xn--p1ai/ который занимается производством и продажей дорожной техники для ремонта и содержания автомобильных дорог. Гарантия, бесплатный сервис, доставка по всей России. Собственное конструкторское подразделение, сервисная поддержка 24/7 – и многое другое для наших клиентов. Подробнее на сайте.
Found a captivating read that I’d like to recommend to you https://club2108.ru/forum/post.php?fid=57
Скачать музыку https://audiobot.pro/ еще никогда не было так просто. Зайдите на сайт, ищите любимые треки или готовые музыкальные подборки, которые вы сможете скачать в один клик без регистрации. Лучшая коллекция mp3 в интернете на сайте. Каждый найдет для себя необходимый жанр и музыку. Скачивайте вашу музыку из ВК в телеграм, на компьютер, телефон или планшет.
Pingback: co trimoxazole double strength bactrim ds
http://kvadrociklov-prokat.ru/
Красные сушеные мухоморы на заказ c доставкой по России на сайте https://red-muhomor.ru/ это возможность купить высококачественные сушеные мухоморы для исследовательских, образовательных и декоративных целей. Зайдите в наш каталог, чтобы узнать больше о наших продуктах. Подробнее на сайте.
buy tiktok followers and likes buy 1000 tiktok followers cheap
https://besuchszweck.org/
can you buy followers on tiktok to go live https://www.templates.com/blog/how-to-get-more-followers-on-tiktok-expert-tips/
To play the Aviator Game, you need a device with internet access and a web browser that supports HTML5. This
fortune tiger
https://lavirgule.news/
http://atvbizon.ru/
https://interventionist.us/
Key Developers and Companies Involved
tigrinho demo
Found a captivating read that I’d like to recommend to you https://wallazz.com/blogs/203320/Получите-Диплом-в-Кратчайшие-Сроки
Центр «Доверие» приглашает вас воспользоваться услугами. Запишитесь к психологу по номеру телефона на сайте. Семейный психолог старается сделать так, чтобы консультации были комфортными для обоих супругов. https://ack-group.ru – сайт, где вы узнаете более детальную информацию. Подростковый психолог выяснит уровень вашей проблемы и подберет подходящий способ ее решения. Цена консультации психолога доступна любому. Психолог в СПБ поможет урегулировать отношения с окружающими и с собой. Обращайтесь!
На сайте https://lan-union.ru предлагается к покупке сетевое и кабельное оборудование. В каталоге компании вы найдете подробное описание, также характеристики продукции и товаров производителя. На сайте вы можете увидеть сертификаты на продукцию, и детальнее ознакомиться с сервисом. Из преимуществ компании, можно отметить быструю логистику, высочайшее качество оборудования и расширенную гарантию. Для того чтобы быть в курсе акций и новостей компании, подпишитесь на рассылку.
Pingback: valacyclovir for herpes
User Experience and Reviews
jogo do tigrinho
Ищите тексты песен? Зайдите на сайт https://txtsong.ru/ где собрано более 185 000 текстов песен от 28 00 исполнителей. Пополнение базы новинками происходит каждый день. Любые группы, любые исполнители – все тексты песен для вас. Подробнее на сайте.
На сайте https://dekorativnye-shtukaturki-77.ru/ в огромном многообразии находится декоративная штукатурка. Она необходима для того, чтобы разнообразить интерьер и сделать его более стильным и ярким. Для того чтобы принять окончательное решение, необходимо ознакомиться с каталогом. Штукатурка дает возможность создать удивительные узоры, интересные рисунки, которые станут яркой деталью. Этот материал отличается экологической чистотой, он полностью безопасен для здоровья людей, животных.
Скачать приложения для Андроид бесплатно на сайте https://appstoreandroid.ru/ такие как мессенджеры, социальные сети, экшен и многое другое. Бесплатные приложения для Андроид в огромном количестве позволят найти все самое необходимое для вас.
Зайдите на сайт https://autostrong-m.ru/ где вы сможете воспользоваться легким поиском новых и б/у запчастей с доставкой в России в интернет-магазине Автостронг! Купить автозапчасти легко – вы можете искать по номеру запчасти, по VIN автомобиля, по марке авто. Зайдите в каталог – запчасти представленные там – в наличии с отправкой в кратчайшие сроки. Подробнее на сайте.
линии средств для интимной гигиены каталог IntiLINE
Unique Features of the Aviator Game
tigrinho
See Real – известная компания, специализирующаяся на наружной рекламе и светодиодных технологиях. Предлагаем выгодные цены и привлечем в бизнес новых клиентов. Будем рады обсудить с вами самую смелую идею. Стремимся к долгосрочному сотрудничеству. Оставьте контактные данные, и мы обязательно свяжемся с вами в ближайшее время. Ищете наружная реклама по всему казахстану? Iseer.kz – сайт, где вы сможете узнать еще больше о нас информации, а также сделать заказ звонка консультанта, для расчета стоимости. Профессиональная команда готова к творческим вызовам. Оцените нашу работу!
Известная игра Hamster Kombat обещает оперативный заработок. Запустить ее можете через официального бота в телеграме. Реальными деньгами платить не надо. У игры доброжелательные отзывы, погрузитесь в мир боевых хомяков. https://hamster-kombat-bot.io – сайт, где представлена вся нужная информация о проекте. Вы узнаете, как начать играть в Hamster Kombat. Ознакомиться с информацией можно прямо сейчас. В Хамстер Комбат можете прокачать своего хомяка и зарабатывать, используя Telegram.
http://ek-x.ru/
players globally. The Aviator Game stands out in this crowded market due to its innovative gameplay and the
tigrinho demo
https://livecamsporno.com/
Stumbled upon an interesting article – I suggest you take a look https://sllife.ru/blog/lecortege/dosug-dlya-dzhentlmenov-v-sanktpeterburge/
На сайте https://iz-nerzhaveyki.ru/ вы можете приобрести из нержавеющей стали подводки, фитинги, фланцы, краны и другое. Здесь вы подробнее узнаете про шаровые краны, рукав высокого давления и гибкую подводку. Каталог на сайте позволит подобрать то, что вас интересует. Вся продукция качественная, она представлена на портале с фото и техническими характеристиками. Если у вас возникли вопросы по продукции, обратитесь к нам по телефону на сайте, либо на электронную почту.
Pingback: """doxycycline"" premature ejaculation reddit"
Overview of the Aviator Game Experience
tigrinho demo gratis
[url=https://griezmann-antoine-fr.biz]griezmann-antoine-fr.biz[/url]
play in diablo game here
griezmann
На сайте turkhit.tv можно [url=https://turkhit.tv/serials2023/]турецкие сериалы 2023 онлайн[/url]. Ежедневно добавляются новые серии в отличном качестве с идеальной русской озвучкой. Здесь вы найдете сериалы всех жанров: от мелодрам до триллеров.
Платформа удобна в использовании и не содержит назойливой рекламы. На turkhit.tv представлены как новинки, так и классические сериалы. Не требуется регистрация или оплата, что делает просмотр еще более приятным. Посетите сайт и наслаждайтесь лучшими турецкими сериалами 2023 года.
NGolo Kante https://ngolokante.prostoprosport-ar.com is a French footballer who plays as a defensive midfielder for the Saudi Arabian club Al-Ittihad and the French national team. His debut for the first team took place on May 18, 2012 in a match against Monaco (1:2). In the 2012/13 season, Kante became the main player for Boulogne, which played in Ligue 3.
KissVK https://kissvk.pro/ это скачать музыку бесплатно в формате mp3 или слушать онлайн на сайте. Огромная коллекция треков всех годов и жанров. Новинки 2024 года, ТОП чарты, ТОП музыка, музыкальные подборки. Скачать музыку по настроению, в машину, на природу, для вечеринки в один клик на сайте.
whether at home or on the go.
fortune tiger demo
Хотите купить занимательные сериалы на DVD? Serialexpress вам с этим поможет. У нас есть диски, которых вы ни в одном магазине не найдете. Качество сериалов великолепное, мы точно знаем, что вы останетесь своим приобретением, очень довольны. Товары в доступе по лояльной стоимости, они отлично упакованы. https://serialexpress.ru – сайт, где есть каталог, также тут можно посмотреть условия оплаты. У нас есть накопительная скидочная система. Качество доставки вас удивит. Остались вопросы? Обратитесь за ответами к нашим компетентным менеджерам.
increase the excitement of the game.
tigrinho demo gratis
ferienwohnung tivat
https://www.montenegro-immobilien-kaufen.com
https://lebelligerant.com/
На сайте https://shemi-otopleniya.ru/ вам предлагают качественную гибкую подводку из нержавеющей стали. Здесь вы сможете найти подробные характеристики с фото товара, а также увидеть всю продукцию. Вся гибкая подводка производится из высококачественной нержавейки. На сайте у вас есть возможность найти раздел с акциями и спецпредложениями. Гибкую подводку вы сможете приобрести под заказ самой разной модификации, диаметра и формы. Портал предлагает исчерпывающую информацию о гибкой подводке, ознакомьтесь с ней в любое удобное время.
https://polskikompas.com/
the anticipation building as the plane climbs higher. The key is to cash out at the right moment before the
tigrinho
https://www.livevideochat.xyz/
Kobe Bean Bryant https://kobebryant.prostoprosport-ar.com is an American basketball player who played in the National Basketball Association for twenty seasons for one team, the Los Angeles Lakers. He played as an attacking defender. He was selected in the first round, 13th overall, by the Charlotte Hornets in the 1996 NBA Draft. He won Olympic gold twice as a member of the US national team.
На сайте https://dekorativnye-shtukaturki-77.ru/ в огромном многообразии находится декоративная штукатурка. Она необходима для того, чтобы разнообразить интерьер и сделать его более стильным и ярким. Для того чтобы принять окончательное решение, необходимо ознакомиться с каталогом. Штукатурка дает возможность создать удивительные узоры, интересные рисунки, которые станут яркой деталью. Этот материал отличается экологической чистотой, он полностью безопасен для здоровья людей, животных.
Музыка ВКонтакте https://audiobot.pw/ – слушать онлайн и скачать музыку бесплатно. Скачать, слушать, бесплатно, без установки программ, на компьютер, телефон, планшет, музыка, вк, vk, вконтакте. Лучшие подборки, лучшие чарты. Миллионы треков в mp3 для прослушивания онлайн или скачивания.
casino game.
fortune tiger
https://pornkingcams.com/
Проституция в российской столице представляет собой комплексной и разнообразной проблемой. Хотя это противозаконна правилами, этот бизнес остаётся существенным нелегальным сектором.
Прошлый
В Союзные времена коммерческий секс имела место нелегально. По окончании Советского Союза, в ситуации экономической нестабильной ситуации, проституция оказалась более видимой.
Сегодняшняя состояние
В настоящее время коммерческий секс в городе Москве принимает многообразие форм, от элитных сопровождающих услуг и заканчивая уличного уровня секс-работы. Престижные предложения обычно осуществляются через онлайн, а на улице коммерческий секс располагается в определённых районах города.
Социальные и экономические факторы
Многие женщины вступают в эту деятельность ввиду материальных проблем. Секс-работа может быть заманчивой из-за возможности быстрого дохода, но она подразумевает угрозу здоровью и охраны здоровья.
Правовые Вопросы
Секс-работа в стране нелегальна, и за ее организацию установлены жесткие наказания. Работников интимной сферы зачастую задерживают к административной и правовой наказанию.
Таким образом, игнорируя запреты, проституция существует как сегментом незаконной экономики российской столицы с значительными социальными и законодательными последствиями.
снять проститутку балашиха
Matthew Michael D’Agati serves as the proprietor of Renewables Worldwide, an alternative energy Company in Massachusetts.
A few time period ago, taking a leap of faith, Matthew D’Agati delved into the world of alternative energy, or in a short opportunity began efficiently selling significant amounts of power, primarily at the business sector, working with solar farm developers and local businesses in the “architecture” of their initiatives.
Continuous media just in the markets, led Matt to become a member of a nearby start up 2 time period back, and in a short time, he became their CSO, in charge of all businesses and site advancement, as well as being provided group possession.
During ideal collaborations and sheer services ethical code, Matt D’Agati brought that team from an initial basic-year gains to in excess of a 300% build up in general sales by entire year two. On that premise, RW, a experienced person-purchased business, was organized with charge of creating sustainable stamina systems for an intelligent and more alternative future.
Most exclusively, recognizing there is an untapped market in the promote and an enhanced method to create outcome, RW is one of a select number of enterprises in the United States government to really concentrate on guest acquire, focusing in both retail and commercial sun grind off-take. Personal vision is to initiate a profit system on a regional, regional, national level, offering various inexhaustible focus equipment just in the of RW.
This enthusiasm in your renewable industry remains to inspire and inspire Matthew in moving forward his venture to work with establishments that communicate the similar of selling renewable vitality possibilities for a further inexhaustible upcoming. Matthew brings each in endeavor from Hesser College.
[url=https://rocketreach.co/matt-dagati-email_55407400]The ways consultant stands out against advisor in green energy under the guidance of Matthew D’Agati.[/url]
[url=http://www.pm-anwaelte.at/de/]Green Fuel as a Answer to Power supply Assurance Stresses by matt d’agatiMatt D’Agati[/url] 7_282dd
На сайте https://elektricheskie-kaminy.ru/ представлены электрические камины в огромном ассортименте. Эти конструкции подарят вашему дому незабываемый уют, комфорт. И самое важное, что они прослужат очень долго. Магазин предпринимает все возможное для того, чтобы процесс покупки был очень простым и понятным. Камины не выбрасывают в атмосферу вредные вещества. А для того, чтобы они начали обогревать, не потребуются дрова, а только розетка. Отсутствует открытый огонь, а потому возможность пожара сведена к минимуму.
Pingback: ciprofloxacin and milk
Зайдите на сайт https://trc20casino.com/ где вы найдете Топ крипто казино в России 2024. Обзор, бонусы на депозит. Такие площадки дают возможность использовать цифровые монеты для депозитов и кешаута. Ознакомьтесь с обзорами, как ввести и вывести деньги, как пройти регистрацию и играйте в удовольствие!
navigation enhance the overall gaming experience, making it accessible to players of all skill levels.
tigrinho aposta
Автозапчасти в Беларуси на сайте https://nikey.by/ это возможность купить в ассортименте автозапчасти, которых более 3 000 000, удобный поиск по VIN, а также номеру запчасти или марки автомобиля, а также большое количество различного масла, фильтров, расходников. Можно забрать самостоятельно или воспользоваться быстрой доставкой.
К-ЖБИ производит большой ассортимент продукции отменного качества. Компания уже успела зарекомендовать себя с лучшей стороны. Мы постоянно развиваемся, гарантируем полное соблюдение сроков, приемлемые цены и высочайший уровень сервиса. Осуществляем оперативную доставку. Ищете щебень вторичный разной крупности вторичной переработки? Gbisp.ru – сайт, где вы можете ознакомиться с детальной информацией о нас. Здесь можно прямо сейчас оставить заявку, укажите контактные данные, и мы свяжемся с вами. Вы получите квалифицированную консультацию. С нами выгодно сотрудничать!
На сайте https://podvodka.okis.ru/ у вас есть возможность заказать качественные гибкие подводки из нержавеющей стали. Широкий перечень продукции, такой как сильфонные гибкие подводки и многое другое вы можете заказать на сайте по телефону. Пользователи в считанные минуты смогут купить товар и найти необходимые модели. Меню сайта позволит вам отыскать то, что вас интересует. Также здесь есть таблица на размеры с ценой, руководство по монтажу и технические характеристики подводки с фото.
Pingback: how long does metformin stay in your system
В компания МеталлоДеталь работают грамотные специалисты, которые имеют опыт производства сложных деталей по персональным эскизам, образцам и чертежам. Вы можете получить консультацию по телефону. Задавайте вопросы, и мы с радостью ответим на них. https://metobrabotka.com – сайт, где представлен каталог продукции, вы можете прямо сейчас ознакомиться с ним. Также здесь имеется галерея фотографий нашей компании. По металлообработке мы осуществляем все виды работ. Доставка продукции осуществляется оперативно. Обращайтесь к нам и не пожалеете!
Game Mechanics
fortune tiger demo
Купити ліхтарики https://bailong-police.com.ua оптом та в роздріб, каталог та прайс-лист, характеристики, відгуки, акції та знижки. Купити ліхтарик онлайн з доставкою. Відмінний вибір ліхтарів: налобні, ручні, тактичні, ультрафіолетові, кемпінгові, карманні за вигідними цінами.
сервисы по ремонту айфонов [url=https://iphonepochinka.by/]https://iphonepochinka.by/[/url] .
квартира у моря кипр
Продажа подземных канализационных ёмкостей https://neseptik.com по выгодным ценам. Ёмкости для канализации подземные объёмом до 200 м3. Металлические накопительные емкости для канализации заказать и купить в Екатеринбурге.
The most stunning transsexual shows on Stripchat. Popular trans chat rooms on free live sex cams. Watch, flirt, and have fun with trans cam models [url=https://bestshemalecams.com/]trans cam girls[/url]
Pingback: provigil ghb
Playing the Aviator Game is a thrilling experience. Players place their bets and watch the plane take off, with
tigrinho demo gratis
Lebron Ramone James https://lebronjames.prostoprosport-ar.com American basketball player who plays the positions of small and power forward. He plays for the NBA team Los Angeles Lakers. Experts recognize him as one of the best basketball players in history, and a number of experts put James in first place. One of the highest paid athletes in the world.
ALCOHOL DELIVERY
We are a Toronto-based company that offers dial-a-bottle service and deliver beverages to all parts of the city.
You won’t ever need to leave your house to repurchase alcohol if you choose a reliable [url=https://after-hours-alcohol.ca/]alcohol delivery Toronto[/url] service like ours.
Why? Because we have a variety of alcoholic beverage alternatives for you to pick from.
Explore our online liquor selection, select the kind and brand of alcohol, then click the place order button. Then what?
Your order will be delivered immediately to your home or workplace location.
Ищите скачать музыку бесплатно с ВКонтакте, слушать онлайн на сайте? Зайдите на https://kissvk.pw/ и вы сможете найти огромную коллекцию треков в mp3 на любой вкус. Ваши любимые треки, подборки, различные жанры, песни 2024, песни прошлых лет. Лучшие музыканты и певцы, подборки от редакции. Любые жанры, хиты, редкие песни – все это у нас на сайте.
На сайте https://moresliv.cc/ вы можете скачать бесплатно сливы курсов через торрент. Здесь каждый день добавляются свежие материалы. Обновления быстрые, а поддержка качественная. Воспользуйтесь поиском на сайте, впишите курс по названию либо по автору. Здесь любой найдет для себя большую коллекцию актуальных курсов, доступных без каких-либо затрат для скачивания. Тут собраны марафоны в таких областях, как финансы, программирование, бизнес и другое. Присоединяйтесь прямо сейчас и расширьте свои возможности и знания.
Summary of Key Points
fortune tiger demo
Pingback: gabapentin contraindications
Maria Sharapova https://maria-sharapova.prostoprosport-ar.com Russian tennis player. The former first racket of the world, winner of five Grand Slam singles tournaments from 2004 to 2014, one of ten women in history who has the so-called “career slam”.
Luis Fernando Diaz Marulanda https://luis-diaz.prostoprosport-ar.com Colombian footballer, winger for Liverpool and the Colombian national team . Diaz is a graduate of the Barranquilla club. On April 26, 2016, in a match against Deportivo Pereira, he made his Primera B debut. On January 30, 2022, he signed a contract with the English Liverpool for five years, the transfer amount was 40 million euros.
plane crashes, making it a game of both luck and timing. This dynamic gameplay keeps players engaged and coming
play aviator game
Found captivating reading that I’d like to recommend to everyone http://industrial.getbb.ru/posting.php?mode=post&f=4&sid=a71f1fb304c85ccd37748e3201494ece
Since its inception, the Aviator Game has undergone numerous updates and enhancements. Technological
aviator game
Kevin De Bruyne https://kevin-de-bruyne.prostoprosport-ar.com Belgian footballer, midfielder of the Manchester club City” and the Belgian national team. A graduate of the football clubs “Ghent” and “Genk”. In 2008 he began his adult career, making his debut with Genk.
Mohammed Khalil Ibrahim Al-Owais https://mohammed-alowais.prostoprosport-ar.com is a Saudi professional footballer who plays as a goalkeeper for the national team Saudi Arabia and Al-Hilal. He is known for his quick reflexes and alertness at the gate.
https://fuckingcouple.com
Андрей Суляев – психоаналитик, справится с запутанными и сложными проблемами. Его основная работа, которую он ведет официально, является частная психологическая практика. Консультации осуществляются онлайн, из любой точки мира и в очном формате. https://sulyaev.ru – здесь вы найдете стоимость услуги, посмотрите ее в любое удобное для вас время. Материалы сайта дадут возможность вам лучше в области психологии сориентироваться. Суляев Андрей готов к дополнительным вопросам и с радостью ответит на них. Задавайте их уже сейчас!
Discovered an article that might interest you – don’t miss it! http://mongolsmc.listbb.ru/viewtopic.php?f=1&t=359
Effective Strategies for Beginners
play aviator game
[url=https://muhammad-ali.com.az]muhammad ali apk[/url]
best boxer in the world Muhammad Ali
muhammad ali apk
Quincy Anton Promes https://quincy-promes.prostoprosport-br.com Dutch footballer, attacking midfielder and forward for Spartak Moscow . He played for the Dutch national team. He won his first major award in 2017, when Spartak became the champion of Russia.
Экспертиза ремонта в квартире https://remnovostroi.ru проводится для оценки качества выполненных работ, соответствия требованиям безопасности и стандартам строительства. Специалисты проверяют используемые материалы, исполнение работ, конструктивные особенности, безопасность, внешний вид и эстетику ремонта. По результатам экспертизы составляется экспертное заключение с оценкой качества и рекомендациями по устранению недостатков.
plane ascends, the higher the multiplier. For example, if you bet $10 and cash out at a 2x multiplier, you win
aviator game online
https://semlava.com/
На сайте https://moyuschie-pylesosy.ru/ в огромном выборе находятся моющие пылесосы, которые будут необходимы в каждом доме. К важным преимуществам подобных устройств относят то, что они смогут провести быструю, комфортную уборку любой поверхности, качественно очистят от пыли и различных загрязнений. Устройства эффективно справятся с самыми разными пятнами быстро, эффективно и без проблем. Дополнительно устройство поддерживает чистоту воздуха. Покупая устройство, необходимо ориентироваться на такие параметры, как: мощность, объем бака.
Зайдите на сайт UKBusiness https://ukbusiness.ru/ и ознакомьтесь с полезной информацией о том как расширить границы своего бизнеса, выйти на международный рынок со своими продуктами и услугами. На сайте вы узнаете: про бизнес в Великобритании, как открыть бизнес в Англии – налоги, финансы, иммиграция и визы, недвижимость и многое другое.
На сайте https://www.krasser.ru/met_izdel.html посмотрите телефон завода для того, чтобы воспользоваться такой полезной услугой, как изготовление металлоизделий, конструкций из стали. Для удобства имеется собственный склад металлоизделий. Услуга оказывается по чертежам. Компания выполняет только высококачественные работы и создает детали, выполненные из металла, независимо от сложности и предназначения. Также можно заказать производство эксклюзивных решений. Все товары разрабатываются с учетом требований, предпочтений заказчика. Техническое задание высылайте по почте.
На сайте https://gdz-history.ru/index.html представлены контурные карты, атласы по истории, другая важная и ценная информация, которая поможет расширить мировоззрение, получить новые знания. Всю полезную информацию вы сможете изучить в режиме реального времени, скачать контурные карты абсолютно бесплатно. Перед вами готовые контурные карты за 5, 6, 7, 8, 9 классы. Скачивайте и распечатывайте чистые карты по истории в любое время, чтобы узнать больше нужной, актуальной информации. Вся самая свежая и качественная информация только здесь!
Смелым игрокам посвящается!
1win предлагает отличные условия для игры: бонусы, высокие коэффициенты, разнообразные игры. Рабочее зеркало позволяет входить без проблем. Играю и выигрываю с удовольствием. Советую попробовать 1win! [url=]1win скачать на iphone[/url]
1win – это надежность и удобство. Простая регистрация, щедрые бонусы, быстрые выплаты. Рабочее зеркало всегда доступно. Играю и выигрываю с удовольствием. Советую всем любителям азартных игр!
Ловите сайт где все в лучшем виде! – http://1winapk.kz/
1win букмекерская скачать на андроид
как восстановить пароль в 1win
скачать 1win зеркало ios
1win выводы
1win xyz
ваучер в 1win что это такое
игровые автоматы на деньги 1win
Удачи!
Зайдите на сайт https://apps-betting.ru/ где вы сможете скачать приложения для ставок от лучших букмекерских контор, а также ознакомиться с ТОП 10 лучших приложений для ставок. Установить приложение можно на Android или iOS (айфон), и начать делать ставки на спорт в 1 клик. Почитайте информацию на сайте как выбрать лучшего букмекера и другие полезные советы.
Convenience: Play from the comfort of your home or on the go.
aviator game
Поставки качественной продукции – главная миссия ЭНЕРГОТЕХСТРОЙ 2000. Компания ценит свою деловую репутацию, мы предлагаем адекватные цены нашим покупателям и удобные способы оплаты. Предоставим вам необходимую информацию и обсудим условия выгодного сотрудничества. Ищете ввгнг купить? Best-vendor.ru/product-tag/kabel-vvgng – сайт, где можете прямо сейчас заказать кабель ВВГнг. У нас вы узнаете, сколько доставка занимает времени. Если у вас возникли какие-то вопросы, позвоните нам по телефону. Мы окажем вам помощь в выборе товаров.
Игра на деньги Money Train отличается простым и удобным интерфейсом. Промокоды и бонусы в этом слоте делают игру более выгодной и завлекательной. Если вам по нраву азарт и риск, то Money Train – это то, что вам необходимо! Лудоманы отмечают отличную графику и звуковое сопровождение. безанкор – сайт, где размещена интересная информация об игре, ознакомиться с ней можете в любое удобное для вас время. Вы сможете узнать, какая присутствует в Money Train тематика, и какова в слоте выигрышная сумма. Играйте разумно и ответственно!
games that stood out from traditional casino offerings. Inspired by the concept of high-risk, high-reward, the
aviator game online
Larry Joe Bird https://larry-bird.prostoprosport-br.com American basketball player who spent his entire professional career in the NBA ” Boston Celtics.” Olympic champion (1992), champion of the 1977 Universiade, 3-time NBA champion (1981, 1984, 1986), three times recognized as MVP of the season in the NBA (1984, 1985, 1986), 10 times included in the symbolic teams of the season (1980-88 – first team, 1990 – second team).
Online Platforms Offering the Game
aviator game
Roberto Firmino Barbosa de Oliveira https://roberto-firmino.prostoprosport-br.com Brazilian footballer, attacking midfielder, forward for the Saudi club “Al-Ahli”. Firmino is a graduate of the Brazilian club KRB, from where he moved to Figueirense in 2007. In June 2015 he moved to Liverpool for 41 million euros.
https://strip-cams.com/
https://oglaszam.pl/ads/ppk/
ensures compatibility across various platforms without the need for additional software downloads.
play aviator game
Pingback: does cephalexin have sulfa in it
Khvicha Kvaratskhelia https://khvicha-kvaratskhelia.prostoprosport-br.com Georgian footballer, winger for Napoli and captain of the Georgian national team. A graduate of Dynamo Tbilisi. He made his debut for the adult team on September 29, 2017 in the Georgian championship match against Kolkheti-1913. In total, in the 2017 season he played 4 matches and scored 1 goal in the championship.
На turkhit.tv вы найдете лучшие [url=https://turkhit.tv/]турецкие сериалы онлайн на русском языке[/url]. Наслаждайтесь бесплатным просмотром в отличном HD качестве и с профессиональной русской озвучкой. Здесь собраны сериалы разных жанров и годов, от драм до фэнтези.
Каждый день добавляются новые серии, так что вы всегда будете в курсе последних событий. Всё, что вы так долго ждали, теперь доступно на русском языке. Заходите на turkhit.tv и наслаждайтесь лучшими турецкими сериалами без назойливой рекламы!
Компания «БытовкиСтарт» успешно работает уже более 5 лет. Мы удерживаем наилучшие цены на рынке и осуществляем оперативную доставку. Продукция всегда имеется в наличии. Бытовки произведены из надежных и прочных материалов. Уверены в качестве наших товаров. https://bytovkistart.ru – сайт, где можете прямо сейчас оставить заявку на профессиональную консультацию. Менеджеры все вежливые, они помогут вам с выбором интересующего вас варианта. Если у вас возникли какие-либо вопросы, не стесняйтесь обратиться к нам!
Damian Emiliano Martinez https://emiliano-martinez.prostoprosport-br.com Argentine footballer, goalkeeper of the Aston Villa club and national team Argentina. Champion and best goalkeeper of the 2022 World Cup.
Can I Play the Aviator Game on Mobile?
aviator game
Pingback: what are the dangers of taking lisinopril?
Encountered a captivating article, I propose you read http://forumjustwoman.getbb.ru/viewtopic.php?f=43&t=945
Call to Action for Players
play aviator game
Jack Peter Grealish https://jackgrealish.prostoprosport-br.com English footballer, midfielder of the Manchester City club and the England national team. A graduate of the English club Aston Villa from Birmingham. In the 2012/13 season he won the NextGen Series international tournament, playing for the Aston Villa under-19 team
Kyle Andrew Walker https://kylewalker.prostoprosport-br.com English footballer, captain of the Manchester City club and the England national team. In the 2013/14 season, he was on loan at the Notts County club, playing in League One (3rd division of England). Played 37 games and scored 5 goals in the championship.
game was designed to provide an adrenaline-pumping experience that mimics the thrill of gambling with a twist.
play aviator game
Laure Boulleau https://laure-boulleau.prostoprosport-fr.com French football player, defender. She started playing football in the Riom team, in 2000 she moved to Isere, and in 2002 to Issigneux. All these teams represented the Auvergne region. In 2003, Bullo joined the Clairefontaine academy and played for the academy team for the first time.
продвижение сайтов в москве недорого [url=https://www.prodvizhenie-sajtov-v-moskve115.ru]https://www.prodvizhenie-sajtov-v-moskve115.ru[/url] .
ALCOHOL DELIVERY
We are a Toronto-based company that offers dial-a-bottle service and deliver beverages to all parts of the city.
You won’t ever need to leave your house to repurchase alcohol if you choose a reliable [url=https://after-hours-alcohol.ca/]alcohol delivery Toronto[/url] service like ours.
Why? Because we have a variety of alcoholic beverage alternatives for you to pick from.
Explore our online liquor selection, select the kind and brand of alcohol, then click the place order button. Then what?
Your order will be delivered immediately to your home or workplace location.
Son Heung Min https://sonheung-min.prostoprosport-br.com South Korean footballer, striker and captain of the English Premier League club Tottenham Hotspur and the Republic of Korea national team. In 2022 he won the Premier League Golden Boot. Became the first Asian footballer in history to score 100 goals in the Premier League
На сайте https://kuhonnye-vytyazhki.ru/ представлены в большом выборе кухонные вытяжки, которые положительным образом влияют на качество воздуха в помещении. Дополнительно отлично впишутся в дизайн и станут его яркой деталью. Вытяжки идеально сочетают функциональность и огромный набор ценных опций. Выбирая подходящий вариант, необходимо полагаться на такие параметры, как: размер вытяжки, который больше всего подходит для вашей кухни. Также учтите и мощность устройства. Установлены привлекательные расценки.
The Aviator Game offers multiplayer options that allow players to compete against each other in real-time. This
п»їaviator game
Ищете video summarizer? Go to Chrome web store chromewebstore.google.com/detail/youtube-transcript/jgibaoklabopileepldnlkbbcibhbgmd and download Youtube text, video transcript, video to text, video summary, ChatGPT video summary, Youtube summary. The best app for text transcription of YouTube videos.
На сайте https://elektricheskie-kaminy.ru/ представлены электрические камины в огромном ассортименте. Эти конструкции подарят вашему дому незабываемый уют, комфорт. И самое важное, что они прослужат очень долго. Магазин предпринимает все возможное для того, чтобы процесс покупки был очень простым и понятным. Камины не выбрасывают в атмосферу вредные вещества. А для того, чтобы они начали обогревать, не потребуются дрова, а только розетка. Отсутствует открытый огонь, а потому возможность пожара сведена к минимуму.
На сайте https://laboratoriya-electro.ru/ вы сможете заказать обратный звонок для того, чтобы проконсультироваться по поводу оказания профессиональных услуг по измерениям, испытаниям различного типа электрооборудования. Вы сможете воспользоваться огромным спектром услуг, в том числе, высоковольтными испытаниями, замерами. В лаборатории находится все необходимое высокоточное оборудование. По этой причине все меры абсолютно безопасные, а ваша техника будет полностью отвечать самым высоким требованиям.
Pingback: pregabalin other names
На сайте https://east-usa.com/index.html ознакомьтесь с подробной, актуальной информацией, которая касается карты Соединенных Штатов Америки. Только здесь представлена самая полная информация, которая вам обязательно пригодится для того, чтобы расширить кругозор. Перед вами подробная карта всех автомобильных дорог. Указаны и наиболее популярные достопримечательности, которые обязательно вас заинтересуют. Карта сгруппирована по следующим регионам: Средний Запад, Запад США, Юг США, Северо-восток.
Хотите погрузиться в мир эмоций и захватывающих историй? Заходите на turklife.tv и смотрите [url=https://turklife.tv/dramy/]турецкие сериалы драмы[/url]! Здесь вас ждут лучшие драматические сериалы в HD 1080 качестве и с профессиональной русской озвучкой. Турклайф ТВ предлагает широкий выбор драм на любой вкус, от новинок 2024 года до классических хитов.
Каждый день добавляются новые серии, и всё это абсолютно бесплатно и без рекламы. Откройте для себя мир турецких драм на turklife.tv – здесь вы найдете всё, что искали!
Зайдите на сайт компании Эквивуд https://equiwood.ru/ которая занимается подбором, доставкой и наладкой деревообрабатывающего оборудования под требования клиента. Только проверенные поставщики и исключительно личные представители на территории стран Азии и Европы, что гарантирует качество оборудования. Осуществляем пусконаладочные работы, обучаем работе на станке, а также гарантия от нашей компании и пост гарантийное техобслуживание.
Opened up an enthralling read – I’d like to share it with you http://repetitor.ekafe.ru/faq.php
Fair Play Policies and RNG
aviator game demo
ForgeHaus – известная компания, которая предлагает широкий спектр услуг: от создания функциональных автонавесов до эксклюзивных декоративных элементов из разнообразных материалов. Мы реализовываем самые смелые проекты. Ищете навес 3 метра? Forgehaus.ru – сайт, где представлена информация о нас, рекомендуем ознакомиться с ней. Гарантируем высочайшее качество нашей работы, индивидуальный подход, приемлемые цены и оперативное выполнение заказов. Продукция вся безопасна и долговечна. Позвоните нам уже сейчас. Ваши идеи превратим в реальность!
На сайте https://panoramaburo.ru/landscape-design оставьте заявку на услугу по ландшафтному дизайну под ключ. Вам предложат интегрированный подход к созданию и проектирование современных внешних пространств. Квалифицированная команда воплотит все ваши мечты и фантазии в реальность. Подробнее ознакомиться с услугами компании, а также галереей работ, можно на сайте. Учитываются особенности каждого участка, предпочтения и пожелания клиентов, убедитесь в этом лично.
Вам интересны последние новости Ленинградской области и Санкт-Петербурга? Nowosti.spb.ru – это то, что вам действительно нужно! Здесь имеются следующие категории: общество, культура, жкх, медицина, спорт, политика, экономика. Предлагаем читателям все в понятном виде и выделяем самое основное. За каждым заголовком и фотографией располагается сюжет. Ищете новости сегодня санкт-петербург? Nowosti.spb.ru – здесь вы найдете много полезной информации. Наш ресурс выбирают те люди, которые ценят наполненность и свежесть. Читайте у нас самые актуальные новости. Рады вам в любое время!
[Casino Name], each offering unique bonuses and promotions for Aviator Game players.
play aviator game
Antoine Griezmann https://antoine-griezmann.prostoprosport-fr.com French footballer, striker and midfielder for Atletico Madrid. Player and vice-captain of the French national team, as part of the national team – world champion 2018. Silver medalist at the 2016 European Championship and 2022 World Championship.
In January 2010, Harry Kane https://harry-kane.prostoprosport-fr.com received an invitation to the England U-team for the first time 17 for the youth tournament in Portugal. At the same time, the striker, due to severe illness, did not go to the triumphant 2010 European Championship for boys under 17 for the British.
Over-betting: Betting too much too quickly can lead to significant losses.
aviator demo game
FinandCrypto.com provides relevant and useful information on the topic of cryptocurrencies, finance, technology. Talking about blockchain in simple words. You can view the content at any time convenient for you. https://finandcrypto.com – a well-known site that is valued primarily for its simplicity and clear interface. Exchange rates are available here. There is also a search here, we recommend using it. Our main task is to help you accept financial and investment solutions. With us you will be aware of the latest news!
Pingback: keflex 500mg for urinary tract infection
Karim Mostafa Benzema https://karim-benzema.prostoprosport-fr.com French footballer, striker for the Saudi club Al-Ittihad . He played for the French national team, for which he played 97 matches and scored 37 goals.
Achraf Hakimi Mou https://achraf-hakimi.prostoprosport-fr.com Moroccan footballer, defender of the French club Paris Saint-Germain “and the Moroccan national team. He played for Real Madrid, Borussia Dortmund and Inter Milan.
Online casino games have surged in popularity due to their convenience and the immersive experience they offer.
aviator game online
Перетяжка мягкой мебели
[url=https://bmx.by/forum/messages/forum5/topic2818/message4694/?result=new]https://bmx.by/forum/messages/forum5/topic2818/message4694/?result=new[/url] .
ремонт айфонов минск [url=https://www.iphonepochinka.by]ремонт айфонов минск[/url] .
The Aviator Game is an exciting online casino game that has captivated players worldwide with its unique blend
aviator game online
Player Reviews and Testimonials
aviator game online
Kylian Mbappe Lotten https://kylian-mbappe.prostoprosport-fr.com Footballeur francais, attaquant du Paris Saint-Germain et capitaine de l’equipe de France. Le 1er juillet 2024, il deviendra joueur du club espagnol du Real Madrid.
Jogo do Tigre https://jogo-do-tigre.prostoprosport-br.com is a simple and fun game that tests your reflexes and coordination. In this game you need to put your finger on the screen, pull out the stick and go through each peg. However, you must ensure that the stick is the right length, neither too long nor too short.
Mohamed Salah Hamed Mehrez Ghali https://mohamed-salah.prostoprosport-fr.com Footballeur egyptien, attaquant du club anglais de Liverpool et l’equipe nationale egyptienne. Considere comme l’un des meilleurs footballeurs du monde
CVzen to najlepsza usługa pisania CV https://cvzen.pl/ i coach kariery dla milionów osób poszukujących pracy na całym świecie. Nasz zespół ekspertów jest zaangażowany w Twój sukces i będzie Cię wspierał przez całą Twoją karierę, od napisania profesjonalnego CV i listu motywacyjnego z uwzględnieniem specyfiki branży, po optymalizację profilu LinkedIn i zapewnienie profesjonalnego coachingu kariery. Odblokuj nowe możliwości dzięki CV, które pokaże Twój potencjał.
advances, players can expect even more exciting features and enhancements to the gameplay experience.
aviator game
Came across a unique article Р it’s worth your attention https://poetzinc.com/read-blog/11090
Declan Rice https://declan-rice.prostoprosport-fr.com Footballeur anglais, milieu defensif du club d’Arsenal et de l’equipe nationale equipe d’Angleterre. Originaire de Kingston upon Thames, Declan Rice s’est entraine a l’academie de football de Chelsea des l’age de sept ans. En 2014, il devient joueur de l’academie de football de West Ham United.
На сайте https://geo-gdz.ru/ представлены ГДЗ по географии, истории. Перед вами огромная библиотека, которая работает в режиме реального времени. Здесь вы найдете школьные учебники, контурные карты, а также атласы, которые помогут получить новые знания, освоить нужную дисциплину. Список всей важной и нужной информации находится прямо на страницах сайта. Каждый год вносятся изменения в соответствии с политическими действиями. Этот сайт предназначен специально для тех, кто не в курсе этих изменений, но стремится расширить свой уровень знаний.
звуковое оборудование для конференц зала [url=https://oborudovanie-konferenc-zalov11.ru]звуковое оборудование для конференц зала[/url] .
Declan Rice https://declan-rice.prostoprosport-fr.com Footballeur anglais, milieu defensif du club d’Arsenal et de l’equipe nationale equipe d’Angleterre. Originaire de Kingston upon Thames, Declan Rice s’est entraine a l’academie de football de Chelsea des l’age de sept ans. En 2014, il devient joueur de l’academie de football de West Ham United.
Читайте реальные отзывы клиентов и сотрудников об агентстве Topface Media на сайте https://topfacemedia-otzyvy.ru/ Поделитесь вашими собственными впечатлениями, оставляйте отзывы о взаимодействии с компанией Топфейс Медиа: дайте оценку нашей работе по управлению репутацией или задайте любой интересующий вопрос.
Thibaut Nicolas Marc Courtois https://thhibaut-courtois.prostoprosport-fr.com Footballeur belge, gardien de but du club espagnol du Real Madrid . Lors de la saison 2010/11, il a ete reconnu comme le meilleur gardien de la Pro League belge, ainsi que comme joueur de l’annee pour Genk. Triple vainqueur du Trophee Ricardo Zamora
На сайте https://pacific-map.com/index.html вы ознакомитесь с картой США с городами, штатами. Перед вами самая полная карта автомобильных дорог США. Есть карта Атлантического, Тихоокеанского побережья, имеется физическая карта США. Только здесь указана самая полная и содержательная информация, которая прольет свет на многочисленные вопросы. Вся информация является содержательной, интересной, актуальной, а потому на нее точно можно положиться. Обращайтесь и вы сюда за информацией, если необходимо что-то уточнить.
Pingback: alcohol and amoxicillin
Found an enthralling article, I recommend you to read http://ukrevent.ru/onlayn-psihologiya-kursyi-ot-nachinayushhih-do-ekspertov/
[b]Здравствуйте[/b]!
Хочу рассказать о своем опыте по заказу аттестата пту, думал это не реально и стал искать информацию в сети, про купить диплом в копейске, купить диплом нового образца, купить диплом с реестром, купить диплом логиста, купить диплом в орле, постепенно вникая в суть дела нашел отличный материал здесь https://1001vieclam.forumvi.com/t7514-topic#8160 и был очень доволен!
Теперь у меня есть диплом столяра о среднем специальном образовании, и я обеспечен на всю жизнь)
Хорошей учебы!
Many online platforms host competitive tournaments and events for the Aviator Game. These events offer players
aviator game
Ищете оклейка авто? Зайдите на сайт just-vinyl.ru где вы сможете узнать все о наших услугах от Just Vinyl. Специализируемся на услуге оклейка авто под ключ, а также в продаже антигравийная пленка. Пленка на авто позволит полностью изменить цвет вашего автомобиля или создать уникальный дизайн с помощью наших услуг по оклейке авто. Ознакомьтесь с ценами, нашими работами и ассортиментом на сайте. Качество в Just Vinyl на первом месте!
Компания ОптимаСтройКомплект имеет прекрасную репутацию. Предлагаем строительные материалы по привлекательным ценам. Сэкономим ваши деньги! У нас предусмотрены скидки крупным клиентам. Можете оплачивать товары любым удобным для вас способом. https://optimalstroy.ru – сайт, где вы найдете больше информации о нас. Здесь можно ознакомиться с продукцией и условиями доставки. Почитайте полезные рекомендации. Наши специалисты всегда вам помогут в случае, если у вас возникнут вопросы. Работаем для тех людей, которые ценят свое время!
Glory Casino
Tomer.ru – сайт, где можно найти подборку самых лучших фильмов о страсти и любви. Посмотрите их обязательно. Они украсят ваш вечер, и даже, смогут повлиять на вашу жизнь. Ищете турецкие сериалы о любви и страсти? Tomer.ru/blog/zhizn-v-turtsii/597-turetskie-serialy-na-russkom-yazyke.html – здесь вы узнаете, почему у российских зрителей турецкие сериалы пользуются популярностью. Подготовили список новинок, их можно смотреть онлайн. Заходите на наш портал, вас ждет много чего интересного и полезного!
Edu.vdgb.ru – сайт, над которым мы каждый день работаем, чтобы он еще лучше стал. У нас вы сможете отыскать много чего интересного. Узнаете про видеокурсы «Фирмы 1С». Развивайте свой мозг и приобретайте новые знания. Курсы 1С онлайн-обучение дают возможность разобраться во всех изменениях и освоить управление программой. С отзывами и ценами можете ознакомиться на нашем ресурсе. https://edu.vdgb.ru – здесь также вы можете приобрести курсы. Вы обретете возможность карьерного роста и в совершенстве научитесь пользоваться 1С. Успейте записаться на онлайн обучение!
На сайте https://deliveryplay.ru/arenda-invalidnyh-kolyasok закажите обратный звонок для того, чтобы воспользоваться такой нужной и полезной услугой, как прокат инвалидных колясок. Вы сможете воспользоваться оперативной доставкой, в том числе, экспресс. Для того чтобы воспользоваться арендой, не нужно вносить залог. Предусмотрен и самовывоз. Полный перечень услуг для клиентов. Действуют привлекательные расценки. Все коляски проходят обработку. Имеются самые разные размеры, независимо от веса.
Калькулятор Ба Цзы https://xn--80aaczlbgb1auuk9b6de5c.xn--p1ai/ позволяет строить карту рождения, она состоит из набора восьми иероглифов, который формируется на основе даты и времени рождения человека, с учётом места рождения. Карта рождения отражает врожденные особенности характера личности сильные и слабые стороны, а также конкурентные преимущества. Она также помогает определить, качество жизни человека в разных сферах, а также определить какие даты будут оптимальными для различных событий, таких как переезд, смена работы, свадьба, начало диеты и другие.
Pingback: letrozole vs tamoxifen