Novodiax research scientist Shuo Chen, PhD recently presented a poster on rapid immunocytochemistry (ICC) at the 109th annual United States and Canadian Association of Pathology (USCAP) meeting in Los Angeles. This presentation was the culmination of a year-long investigation into the use of Novodiax polyHRP-conjugated antibodies (ihcDirect®) for novel applications in rapid immunocytochemistry (ICC).
There’s a lot of excitement surrounding this work, as the concept of “rapid” ICC testing doesn’t really exist on the market today, although the technology holds great potential for researchers and clinicians alike.
Following the meeting, we had a chance to catch up with Shuo to learn more about the poster presentation and Novodiax’s development efforts in the area of rapid ICC. Below is a summary of our conversation:
What is immunocytochemistry (ICC)?
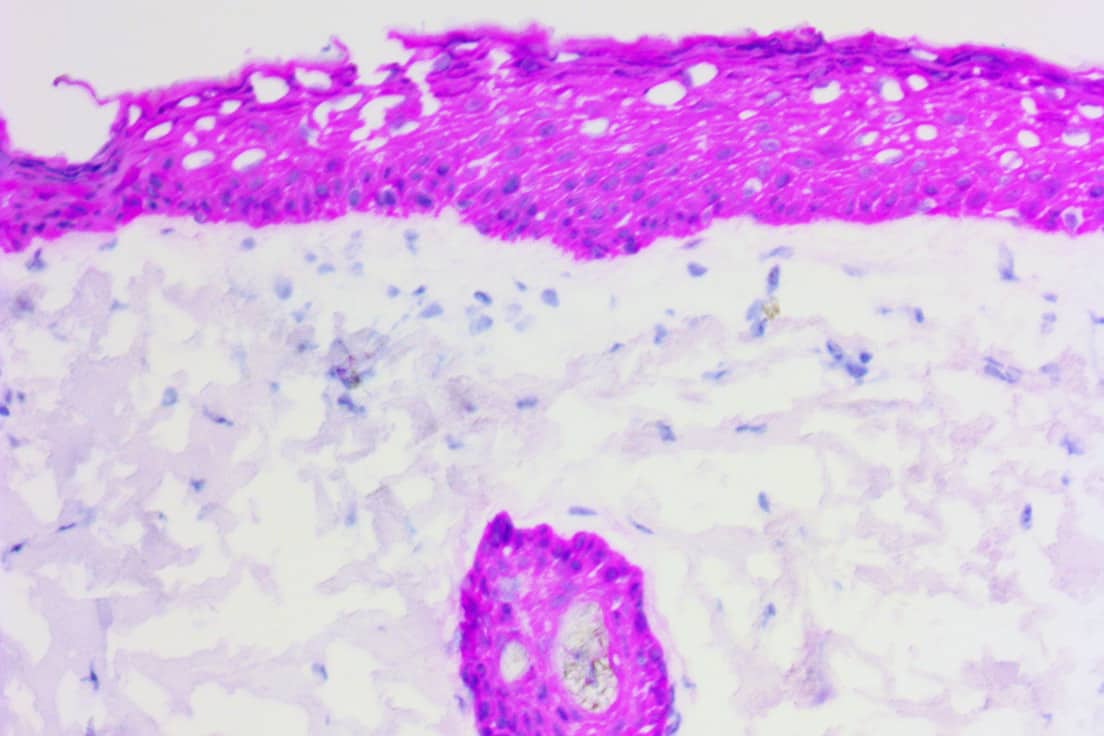
ICC is an immunoassay (a special stain) that allows you to resolve molecular targets (i.e. antigens) in biological specimens with a great deal of sensitivity using antibodies. Imagine that you are a researcher or clinician and you have a sample of cells where you are struggling to differentiate T cells from B cells based on morphology alone. That’s where a CD20 antibody and ICC staining could come in handy. The literature shows that B-cell lymphomas like diffuse large B-cell lymphoma (DLBCL) and Burkitt’s lymphoma typically stain positive for the CD20 biomarker while T-cell lymphomas like anaplastic large-cell lymphoma (ALCL) are generally CD20-negative1.
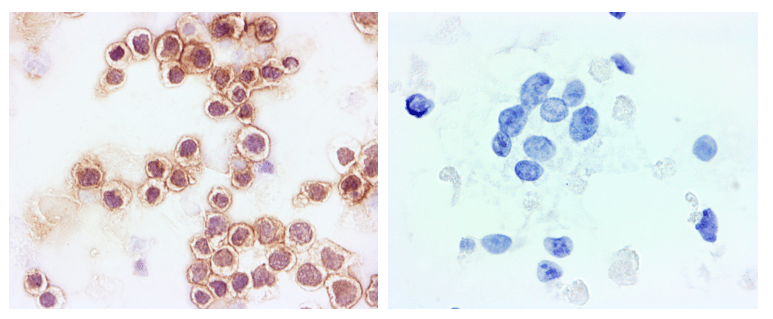
Figure 1. B Cell vs T Cell Lymphoma. CD20 positive B-cell lymphoma (left) and CD20 negative T-cell lymphoma (right)
What is the benefit of a rapid ICC stain?
Immunoassays have historically been time-consuming procedures, although more recent advances in polymerized horseradish peroxidase (polyHRP) and direct IHC have opened up a new world of applications. The Novodiax ihcDirect® family of IVD immunohistochemistry (IHC) products, for example, allow users to complete IHC staining on frozen sections in as short as 10-minutes2. Such rapid IHC assays have been shown to enable timely intra-operative evaluation of resection margins (e.g. Mohs micrographic surgery3) as well as intra-operative differential diagnosis (e.g. breast cancer surgery4). There is now a lot of interest in similarly fast and sensitive biomarker-based cytology tests. One potential application that we’ve seen requests for is 10-20 min. ICC tests for rapid on-site evaluation (ROSE) of fresh whole cell specimens. Physicians have told us that this may help them determine sample adequacy and triage biopsy material appropriately on the spot without having to wait for cell block preparations. That could go a long way towards improving patient care and reducing waste / costs.
Tell us a little bit about the studies you conducted leading to this poster presentation.
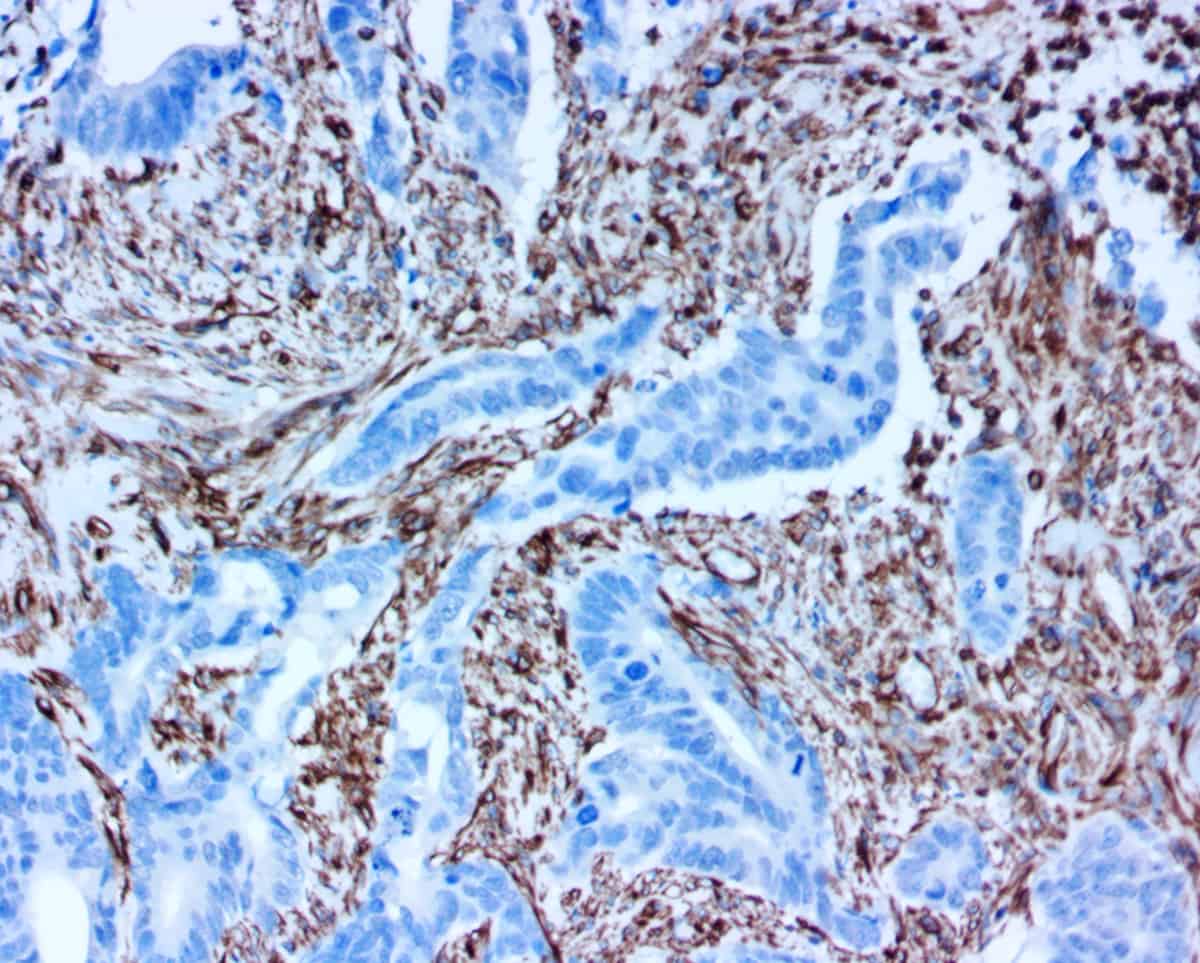
We actually started investigating rapid ICC many years ago. Novodiax’s polyHRP-conjugated antibodies worked very well for both IHC staining of tissues and ICC staining of cells from the get go. At that time, however, we received immediate requests from physicians for IHC tests and as a result proceeded with development of IVD tests for IHC. We continued to hear from cytopathologists and interventional radiologists who were seeking fast ICC stains for cellular specimens. So, for about the past year, we have seriously been diving in to research and characterize novel applications of Novodiax polyHRP-conjugated antibodies for rapid ICC staining, namely on freshly prepared whole cell samples that require minimal prep time. In our studies, we evaluated nine Novodiax polyHRP antibodies (AE1/AE3, CD20, CD45, CK5, CK 8/18, Ki67, Mart-1, Pan-CK 4Abs, and SOX10) on a wide variety of cell specimens (cheek swabs and cell cultures) using multiple fixative types (from alcohol to acetone and formalin). In the end, we were able to demonstrate that ICC staining can be successfully completed on fresh whole cell specimens within 10-20 minutes using Novodiax polyHRP-conjugated antibodies!
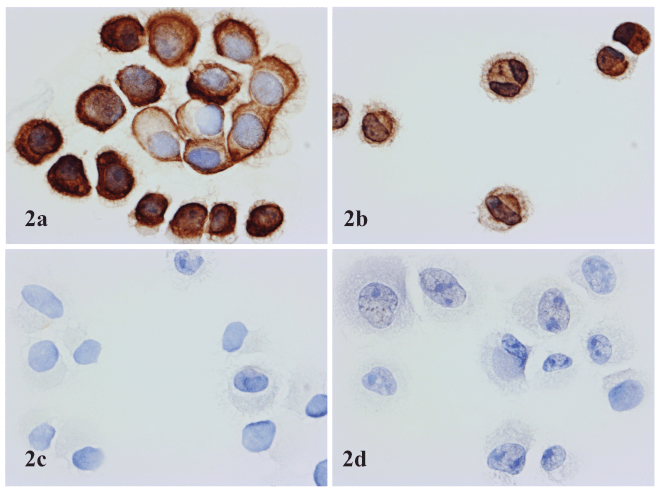
Figure 2. Application of Novodiax ihcDirect Pan-CK 4Abs on fresh cell samples. 2a: SK-BR-3 cell line (mammary gland/breast, derived from metastatic site); 2b: SiHa cell line (cervix, squamous cell carcinoma); 2c: SK-MEL cell line (skin, malignant melanoma); 2d: A375 cell line: (skin, malignant melanoma). 2a and 2b show positive staining, while 2c and 2d show negative results.
What questions/comments did USCAP meeting attendees have about your poster?
The attendees had some very sharp and insightful questions. One of the most common questions I received was concerning validation of rapid ICC assays, especially for use in CLIA labs. There is a long history, tried-and-true techniques, and extensive literature addressing the clinical use of IHC testing. But we are just now starting to build some notions of what a gold standard / reference for a rapid ICC test on fresh whole cell specimens (not just cell blocks) would look like. We also had some great conversations about the clinical utility of rapid ICC assays, including factors that may initially serve as barriers to adoption.

What are the next steps for your investigation into rapid ICC?
As mentioned, validation on real-world samples is critical for this technology to advance to the next stage. We also need to better understand the impact of different fixatives and fixation times on staining results. There are definitely some differences when compared to using the same ihcDirect antibodies on tissue sections vs cell specimens. Additional studies are also needed for assessing other available Novodiax polyHRP-conjugated antibodies beyond those included in this study, and determination of optimal working conditions for other widely used fresh clinical cytology specimens (e.g. fine needle aspirates, core needle biopsies, blood smears, touch imprints, etc.)
→ WOULD YOU LIKE TO COLLABORATE ON A RAPID ICC STUDY? CONTACT US TODAY!
What other interesting things did you see at USCAP that may shape your future research efforts?
Dr. Sharon Song and Dr. Jalal Jalaly at the University of Pennsylvania presented a very interesting poster evaluating the correlation between IHC on tissue sections vs ICC on cell blocks using p16 antibodies. Although our own testing was done on whole cell specimens, this sort of work will be invaluable in helping establish standards for ICC testing. It was also exciting to see the growth of investigations into the application of machine learning algorithms to pathology specimens.The future appears bright!
[1] Jacobsen, Eric. Anaplastic Large-Cell Lymphoma, T-/Null-Cell Type. The Oncologist July 2006 vol. 11 no. 7 831-840.[2] Liu, Mei et al. A Direct Immunohistochemistry (IHC) Method Improves the Intra-operative Diagnosis of Breast Papillary Lesions Including Breast Cancer. Discov Med 28(151):29-37, July 2019. [3] Sroa N, Campbell S, Ravitskiy L. Immunohistochemistry in mohs micrographic surgery: a review of the literature. J Clin Aesthet Dermatol. 2009;2(7):37–42. [4] Liu, Haiyan. Application of immunohistochemistry in breast pathology a review and update. Arch Pathol Lab Med. Vol 2a 2b 138:1629-1642. Dec 2014.